Self-assembly method for two-dimensional mesoporous materials: a review for recent progress
Abstract
Two-dimensional mesoporous materials (2DMMs) refer to thin two-dimensional (2D) nanosheets with randomly dispersed or ordered mesopores, which can combine the advantages of 2D materials and mesoporous materials while overcoming their inherent drawbacks, leading to enhanced application performance. A self-assembly strategy has been recognized as a promising manufacturing method for 2DMMs with customized performance. Over the past decades, encouraging progress has been made in the development of 2DMMs via the self-assembly strategy with a variety of compositions, morphologies, mesoporous structures, and pore sizes. Here, we provide a comprehensive review on recent progress in the fabrication of 2DMMs through this strategy, focusing on the synthesis methods, including molecular self-assembly methods, single micelle assembly methods, multi-templates methods, surface-limited co-assembly methods, and template-free methods. In addition, we set out the challenges faced by 2DMMs in future research and point out potential development directions.
Keywords
INTRODUCTION
Functional mesoporous materials have attracted widespread attention due to their merits of high surface area, large pore volume, tunable nanostructures, and diverse compositions. They have broad application potential in catalysis[1,2], energy storage[2-5], gas adsorption separation[6], sensing[7], and other fields[8-10]. The mesoporous materials obtained by traditional strategies are generally bulk materials, which are three-dimensional ordered assemblies with nanopores. Their internal active sites cannot be fully exposed and are not conducive to the rapid transport of guest molecules in the pores, which greatly limits their application performance[11,12]. Currently, mesoporous materials can be extended to multilevel architectures from 0 dimensions to 3 dimensions through bottom-up self-assembly[13]. Despite the fact that considerable progress has been made in synthesis methods, it remains a challenge to accurately and directionally design and synthesize mesoporous materials based on the relationship between structure and properties to meet the needs of increasingly diverse applications.
Two-dimensional (2D) nanomaterials are a new kind of anisotropic sheet material with lateral dimensions ranging from several hundred nanometers to several micrometers[14]. Since the first exfoliation and characterization of single-layer graphene in 2004[15], 2D materials have gained extensive interest due to their remarkable mechanical, thermal, electrical, magnetic, and optical properties[16,17]. There have been qualitative new members of this family, such as transitional metal dichalcogenides[18], layered double hydroxides[19], and transition metal carbides and nitrides (MXenes)[20], and their applications range from fundamental studies to electronic and photonic devices. However, 2D materials tend to form dense stacked structures, which greatly hinder their mass transfer processes and the full utilization of active surfaces.
Two-dimensional mesoporous materials (2DMMs) not only possess the structural advantages of mesoporous materials but also have ultra-thin 2D geometric structures, which can fully overcome the shortcomings of traditional bulk mesoporous materials[21,22]. On the one hand, ultrathin 2D morphology with large lateral dimensions and atomic thickness comes into being a high theoretical specific surface area, which facilitates the electric structure regulation. On the other hand, pushing mesoporous materials toward the lateral dimension to form unique 2D nanosheets can effectively solve the drawbacks of bulk materials and greatly improve mass transfer efficiencies[23]. Therefore, it is of great significance to develop 2DMMs.
Previous studies have confirmed that self-assembly is an effective strategy for synthesizing 2DMMs[24,25]. Compared to other synthetic approaches for 2DMMs, such as hard templating methods, a self-assembly method shows incomparable merits in the flexible control of pore sizes, architecture, and wall thickness, which determines the final performance of 2DMMs. Over the past few years, through self-assembly methods, great progress has been made in the development of 2DMMs with a variety of compositions, morphologies, mesoporous structures, and pore sizes. Herein, we briefly review recent progress in the fabrication of 2DMMs, focusing on their synthesis strategies, properties, and underlying mechanisms. In addition, we anticipate potential challenges that 2DMMs may face in future research and identify the potential development directions and opportunities. We hope that through this review, readers can gain inspiration and a better understanding of customizing the synthesis of 2DMMs.
INSIGHTS INTO THE SYNTHESIS OF 2DMMS
2DMMs, including inherently layered or non-layered nanosheets, exhibit attractive properties and superior performance in a variety of application fields and can usually be generated through a “top-down” or “bottom-up” strategy[26]. The top-down approach is one of the most commonly used methods to synthesize 2DMMs. In this case, a 2D structure is first prefabricated and then etched or structurally converted into pores. This method typically produces atomically thin nanosheets with disordered porous and defective structures. Moreover, this approach suffers from complex procedures, low yield, and poor controllability, which limits its application performance[27,28]. On the contrary, bottom-up strategies have high versatility, generally do not require complex operation steps, and provide a pathway to obtain a large number of hybrid and composite materials from inorganic, organic, and even biological starting components[29,30]. Especially, a series of 2DMMs with controllable structures, compositions, and morphology have been constructed at the nanoscale or even molecular level by combining the “bottom-up” synthesis concept with a self-assembly strategy.
In the following section, we have systematically summarized the synthesis work of 2DMMs in recent years; according to different self-assembly synthesis processes, the synthesis methods of 2DMMs are classified into five classes: molecular self-assembly methods, single micelle assembly methods, multi‑templates methods, surface-limited co-assembly (SLCA) methods, and template‑free methods.
MOLECULAR SELF-ASSEMBLY METHOD
It is well known that molecular self-assembly exists widely in nature and living systems. Based on the assembly principle of amphiphilic molecules, various novel functional nanostructured materials, especially ordered mesoporous materials, can be designed and created. This material possesses high surface areas, regular arrangement, and uniform nanopores and exhibits broad application prospects in catalysis, separation, energy, and other fields[31,32]. Due to their unique nanoscale effect, mesoporous materials exhibit excellent properties, such as unique surface acidity, high electronic mobility, and improved coordination catalysis abilities. In addition, these mesoporous materials possess high porosity, which is conducive to the loading and diffusion of ions, molecules, and even nanoparticles within the porous structure, providing rich interfaces and active sites for a variety of applications[33,34].
Molecular self-assembly is an effective method for directly preparing structurally precise nanomaterials and molecular scale materials. Due to its advantages of controllable preparation, precise thickness control, and easy removal of templates without residue, this method provides an ideal platform for the construction of complex and layered 2D structures, such as mesoporous nanosheets[35]. The key issue of the molecular self-assembly method is to establish an interface that allows the micelles to self-assemble in two dimensions; diverse interfacial scales exhibit various characteristics in the directional interfacial assembly of 2DMMs, resembling the classical chemical vapor deposition synthesis of graphene on metal catalysts. The gas-liquid, liquid-liquid, and solid-liquid two-phase interfaces provide infinite space for the 2D growth process[36-38].
For instance, Zu et al. reported a novel layered phase separation behavior using amphiphilic block copolymers [polystyrene b polyethylene oxide (PS-b-PEO)] to form stable terminated layered micelles (i.e., nanoconfined self-assembly strategies) with precursors in a layered colloidal confined nanospace[39] [Figure 1]. The fabrication of ordered mesoporous iridium (Ir)-IrOx/C catalysts by nano-limited self-assembly has mainly undergone three stages: Firstly, tetrahydrofuran (THF) is preferentially volatilized at
Figure 1. Illustration of the formation of ordered mesoporous lamellar Ir-IrOx/C catalysts via the nanoconfined self-assembly approach. This figure is quoted with permission from Zu et al.[39]. PS-b-PEO: Polystyrene b polyethylene oxide; THF: tetrahydrofuran.
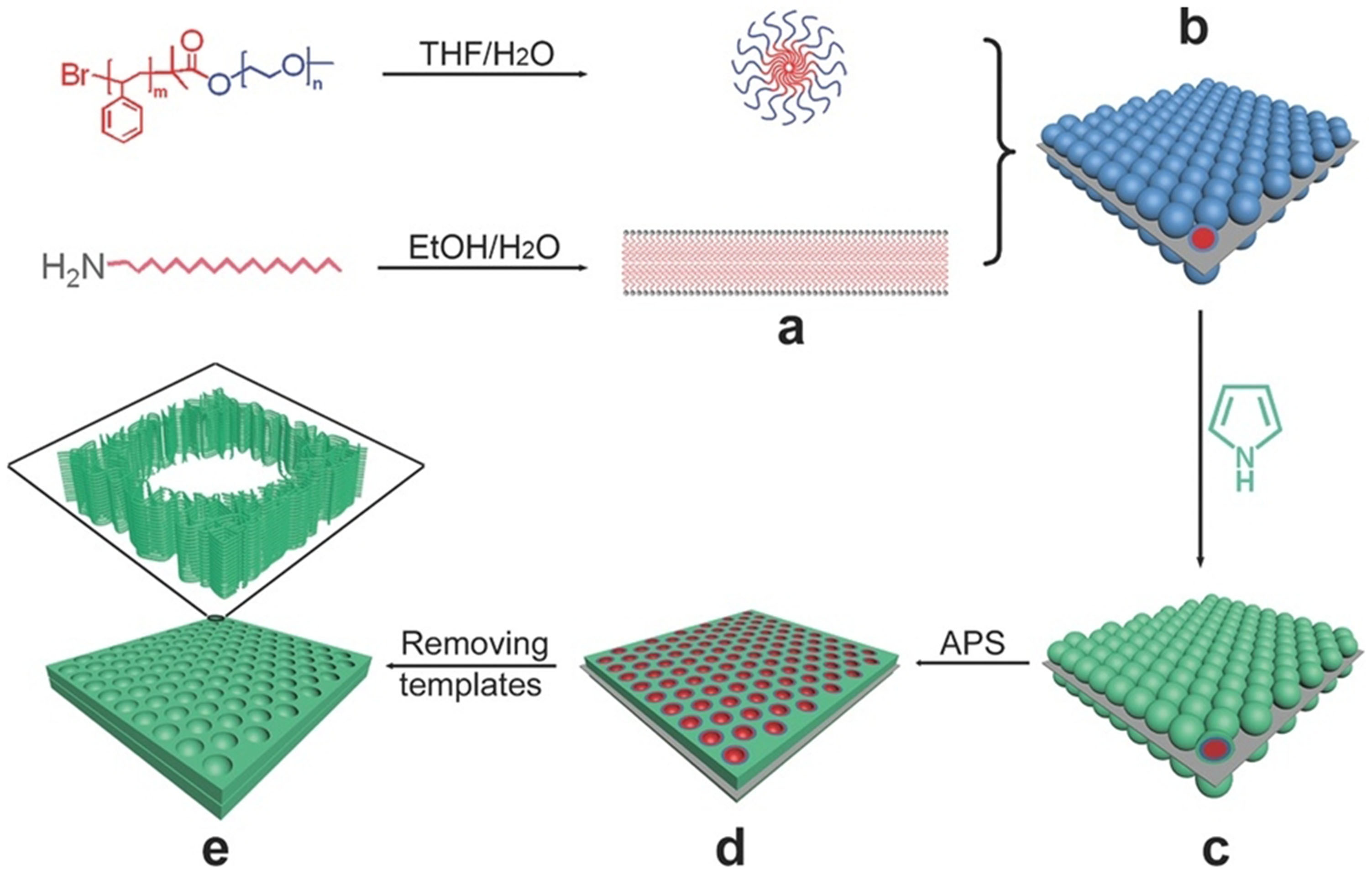
Molecular self-assembly is a highly versatile methodology to nanoarchitect functional systems from various small units. The molecular self-assembly for mesoporous materials usually refers to the commonly used soft template method. The molecular self-assembly method is to introduce an interfacial template into the solution system[40,41]. Through non-covalent action, the copolymer assembly and functional precursor molecules are adsorbed on the interface for co-assembly. After the precursor molecules are cross-linked and the polymer template is removed, 2D porous materials with ordered mesoporous structures are obtained. In addition, the pore structure of the surface, such as spherical pores and cylindrical pores, can be controlled by using different copolymer assembly templates. Lui et al. prepared 2D ultra-thin mesoporous conductive polypyrrole materials with controllable pore sizes (7-14 nm), adjustable thicknesses (25-30 nm), and high specific surface areas (96 m2·g-1) through the collaborative self-assembly of small and large molecules in solution[42] [Figure 2]. This method utilizes long-chain fatty amine (octadecane amine) and amphiphilic block copolymer PS-b-PEO as small and large molecular self-assembly precursors, respectively, which self-assemble in solution to form 2D ultra-thin lamellar structures and monodisperse spherical micelles. At the same time, polymer spherical micelles can spontaneously aggregate and arrange on the 2D lamellar surface formed by fatty amines through non-covalent bonds, such as hydrogen bonds and electrostatic interactions, forming a 2D sandwich structure of supramolecular assemblies. Pyrrole molecules are adsorbed and aggregated into the polyethylene oxide (PEO) phase region of polymer spherical micelles through non-covalent bond forces such as hydrogen bonds. Subsequently, polymerization occurs under the action of an initiator to form a continuous polypyrrole network. After that, using an organic solvent to dissolve and simultaneously remove the fatty amine and copolymer template, a 2D ultra-thin conductive polypyrrole with a regular mesoporous structure was obtained. The 2D ultra-thin sheet layer formed by long-chain octadecane amine can ensure the formation of a 2D structure of the material. The polymer spherical micelles can ensure the fabrication of the mesoporous structure, and the pore size can be tuned via the length of the PS chain segment.
Figure 2. Schematic illustration of the synthesis of the 2D mesoporous PPy nanosheets. This figure is quoted with permission from Liu et al.[42]. APS: Ammonium persulfate; PPy: polypyrrole; THF: tetrahydrofuran; 2D: two-dimensional.
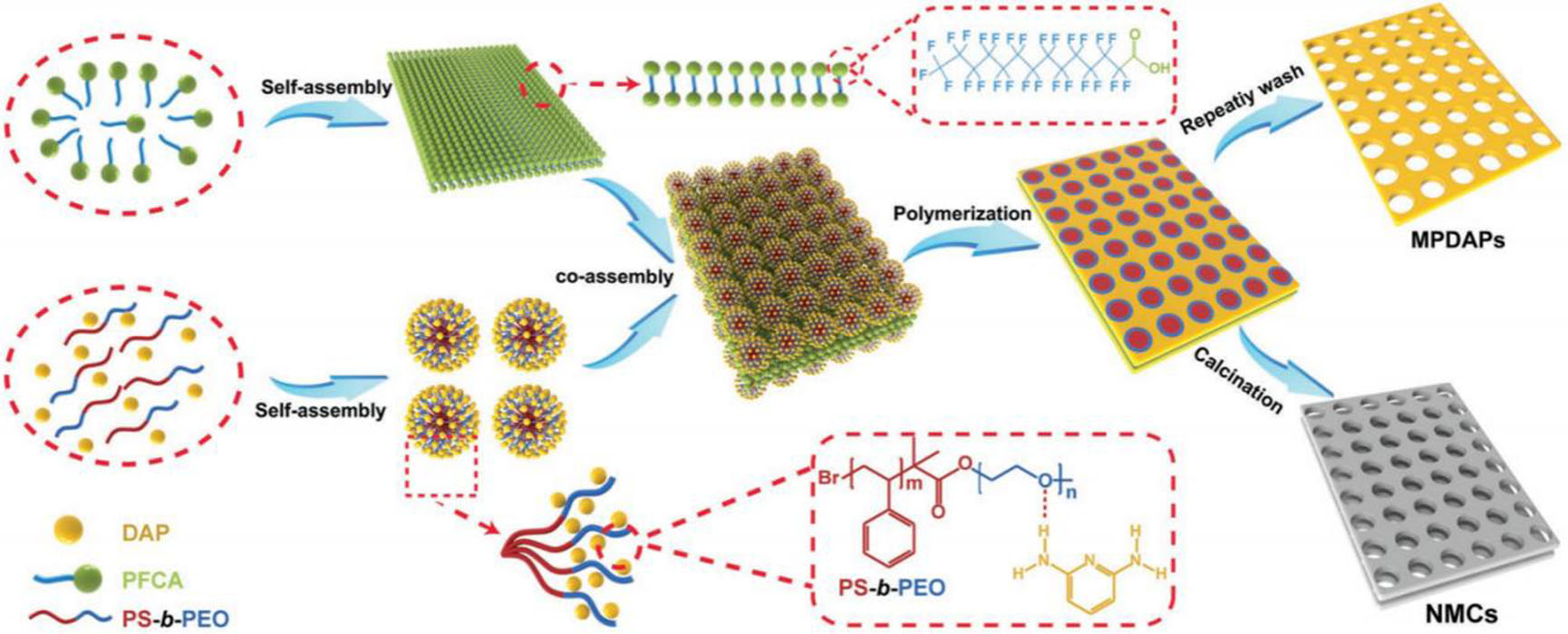
As a kind of lightweight 2D materials with abundant functional groups, 2D polymers show good performance in adsorption, energy storage, and sensors[43-46]. Our group synthesized 2D mesoporous polydiaminopyridines (MPDAPs) through a multi-dimensional molecular self-assembly strategy[47] [Figure 3]. In this strategy, block copolymer polystyrene polyethylene oxide (PS-b-PEO) first self-assembles into zero-dimensional spherical micelles and then combines with DAP molecules through hydrogen bonds to form composite micelles of mDAP/mPS-b-PEO. At the same time, perfluorinated tetradecanoic acid (PFCA) molecules formed 2D organic substrates [mesoporous PFCA (mPFCA)] in mixed solvents. Based on a hydrogen bond, mPFCA is co-assembled with mDAP/mPS-b-PEO and rapidly polymerized into a polymer under the induction of ammonium persulfate. After the removal of PFCA and PS-b-PEO by washing with ethanol and 1, 4-dioxane multiple times, 2D MPDAPs were obtained. By changing the molecular weight of PS-b-PEO, the mesoporous size of MPDAPs can be effectively adjusted. Due to their abundant amino groups and pyridine nitrogen sites, the MPDAPs obtained showed high catalytic activity in the Knoevenagel condensation reaction.
Figure 3. Schematic illustration of the synthetic procedure for MPDAPs and NMCs via a multi-dimensional molecular self-assembly strategy. This figure is quoted with permission from Zhang et al.[47]. DAP: 2,6-diaminopyridine; MPDAPs: mesoporous polydiaminopyridines; NMCs: N-doped mesoporous carbon; PFCA: perfluorinated tetradecanoic acid; PS-b-PEO: polystyrene b polyethylene oxide.
As described above, molecular self-assembly is a general synthesis method for the controlled synthesis of ordered porous structures, which can achieve precise control of the composition, coordination environment, and nanostructure of the designed catalysts. However, due to the poor structural stability of layered micelles of block copolymers, it has been difficult to synthesize ordered 2D layered structures by using layered micelles.
SINGLE MICELLE ASSEMBLY METHOD
For the last few years, detailed research has been conducted on the self-assembly of micelles and skeleton precursors into mesoporous structures at liquid-solid, liquid-liquid, and gas-liquid interfaces to construct functional mesoporous materials with different chemical compositions, 2D morphologies, and mesoporous structures. In comparison with the single-phase solution synthesis method, introducing a two-phase interface in the synthesis environment alters the self-assembly behavior between micelles and skeleton materials, thus making it possible to customize the synthesis of unique mesoporous structures. Furthermore, regulating interfacial tension is essential for controlling the self-assembly process to achieve accurate preparation. Especially in recent years, the breakthrough based on the concept of “single micelle” assembly mechanisms has great prospects and significance for the precisely controlled preparation of functional mesoporous materials[48].
What is single micelle assembly? First, the formation of mesoscopic structures generally goes through four processes: liquid crystal mesoscopic phase, micelle monomer formation, micelles assembly and arrangement, and template agent removal. Although the process has been relatively clear, effective control of the intermediate phase cannot be achieved. After years of exploration, Zhao et al. found that by using a two-solvent volatilization system with a large boiling point difference, it is possible to achieve certain benefits. In this system, it is preferable for the low-boiling solvent to volatilize first, ensuring that the critical micelle concentration is reached. On the other hand, retaining high boiling point solvents can efficiently hinder the further assembly and arrangement of micelles, resulting in relatively free discrete micelle monomers, referred to as single micelles[49]. The single micelle assembly method is to perform subsequent secondary assembly through other inducing forces to achieve single micelle level manipulation[50,51].
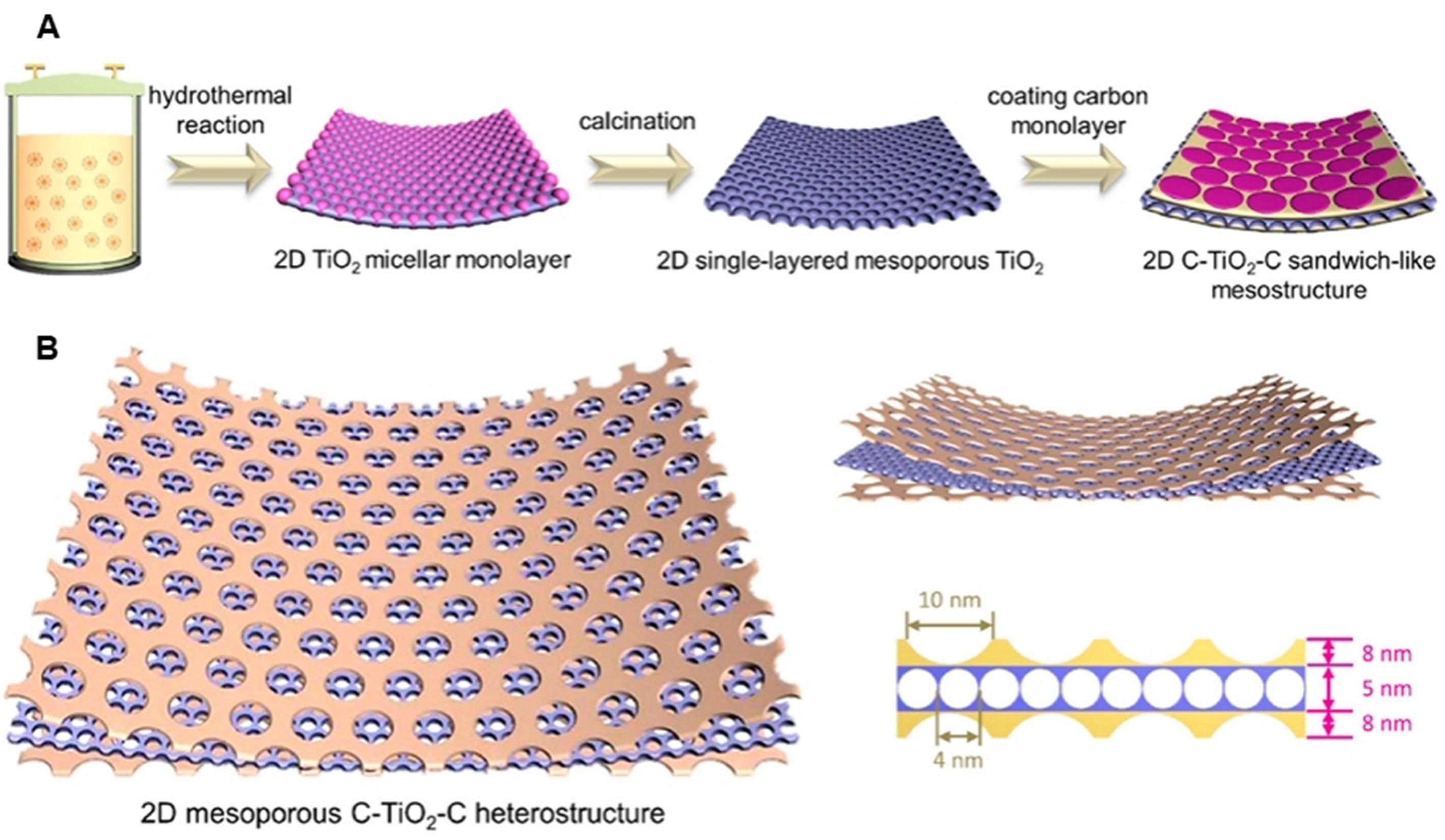
Lan et al. provided a new synthesis strategy for mesoporous heterojunction composite structures, the preparation of ultra-thin single-layer mesoporous structures achieved through precise self-assembly at the level of a single micelle[52] [Figure 4A]. Through the single micelle assembly method, free single micelle monomers are formed in advance as the basic elements to achieve 2D monolayer micelle assembly [Figure 4B]. Combining the limited 2D directional assembly of titanium-based single micelles with the interfacial self-assembly of carbon-based single micelles, a 2D heterogeneous interface is obtained, which ensures the realization of open pores and high contactable specific surface areas, improves the conductivity of titanium oxide materials, and ensures a stable heterogeneous interface during electrochemical storage. Therefore, this 2D heterogeneous junction achieves high-performance pseudocapacitive sodium ion storage.
Figure 4. A single micelles assembly method to 2D monolayered meso-hetero. (A) Preparation procedures of the 2D meso-hero structure; (B) Structure models of the obtained 2D meso-hetero. This figure is quoted with permission from Lan et al.[52]. 2D: Two-dimensional.
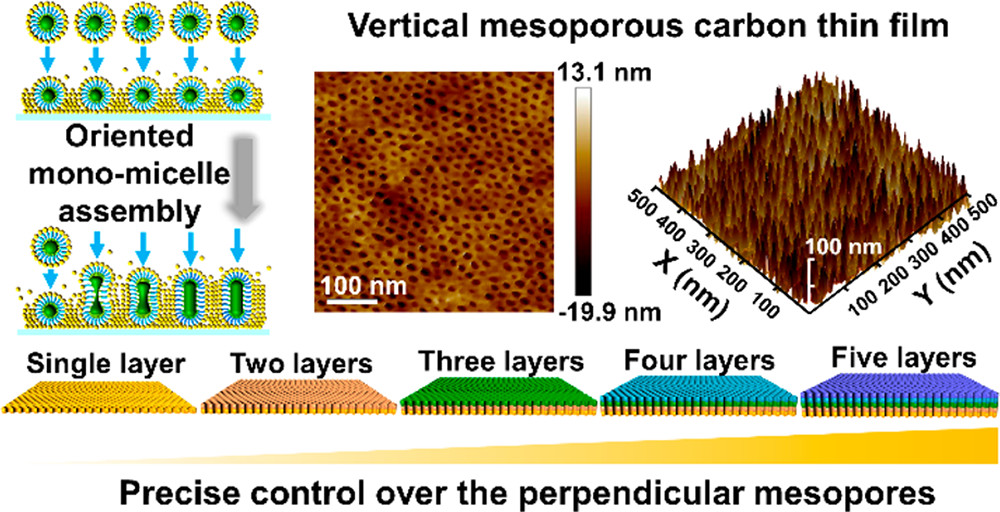
Moreover, the simple and universal single micelle assembly method was employed for precisely controlling the synthesis of vertically ordered mesoporous carbon films at a centimeter scale on multiple substrates[53]. This vertical mesoporous structure can be assembled at the solid-liquid interface, using amphiphilic triblock copolymers as templates, dopamine (DA) as a carbon source, and trimethylbenzene as fusion and swelling agents, and mediating the fusion of single molecule micelle into cylindrical micelles. As shown in Figure 5, the mesoporous carbon film prepared by this method has excellent high mesoporous properties, high specific surface areas (472 m2·g-1), and pore sizes of up to 9.1 nm. This directional assembly process can be highly regulated at the single micelle level, achieving precise thickness control of mesopores from monolayers to multilayers and pore size control from 8.4 to 13.5 nm. Compared to traditional porous carbon membrane sensors, carbon membranes with vertical mesoporous channels have high permeability, which shows a lower detection limit (50 nmol·L-1) and higher DA detection sensitivity.
Figure 5. Schematic illustration of the synthetic procedure for vertically aligned mesoporous carbon thin films with well-ordered mesostructures on various substrates at the centimeter scale. This figure is quoted with permission from Wang et al.[53].
This single micelle assembly method provides a new method for multifunctional materials related to mass transfer and diffusion and also plays a certain role in promoting the precise synthesis of mesoporous materials[54]. However, there is still a long way to go to achieve controllable preparation of crystalline 2DMMs.
MULTI-TEMPLATES METHOD
The multi-templates method refers to the simultaneous use of polymer micelles as mesoporous growth templates and 2D materials as substrate templates during the 2DMMs synthesis process[42,55]. This method is suitable for the preparation of planar mesoporous nanosheets or sandwich-like mesoporous heterostructures, which typically depend on the fabrication of mesoporous inorganic/organic layers on 2D directional substrates[56-58]. Under the force of hydrogen bonding, electrostatic attraction, or coordination interaction between 2D substrates and inorganic/organic precursors, sandwich-shaped precursor template composites were first assembled. Subsequently, after specific post processing, a target sandwich-shaped mesoporous nanosheet is revealed, and a planar mesoporous nanosheet can be observed after clearing away the 2D template. Remarkably, the mesoporous structures arise from growth vacancies, accumulation of nanoparticles, and chemical reactions. To date, graphene oxides (GO), hexagonal boron nitride, graphitic carbon nitride, transitional metal dichalcogenides, layered double hydroxides, and MXene have been proven to be ideal sacrificial 2D templates for the preparation of in-plane mesoporous nanosheets.
As an example, the synthesis scheme of mesoporous polydopamine/MXene (mPDA/MXene) is described in Figure 6A[59]. The thickness of the MXene (Ti3C2Tx) nanosheet is about 1.5 nm. The obtained MXene layer has polar terminal groups of F, O, and OH, which can be surface-modified. Firstly, the P123 copolymer is dissolved directly in water to form a cylindrical micellar under 40 oC. MXene nanosheets and DA hydrochloride were stirred in the micellar solution. The cylindrical micelles were adsorbed on the surface of MXene by hydrogen bonds and electrostatic attraction, while the DA monomers were adsorbed on the PEO crown, resulting in the
Figure 6. (A) Schematic illustration of the synthetic procedure for the mPDA/MXene heterostructure. This figure is quoted with permission from Li
Constructing 2D ordered heterogeneous structures via 2D template methods is an effective way to improve the application performance of materials[60,61]. Recently, our group synthesized 2D ordered mesoporous carbon/titanium carbide heterostructures (OMCTs) in an aqueous system using a triblock copolymer (Pluronic F127) as a template, a low molecular weight phenolic resin as a carbon source, and a Ti3C2Tx nanosheet as an interface[62] [Figure 6B]. The presence of ordered mesoporous carbon can not only inhibit the stacking of Ti3C2Tx nanosheets but also prevent the oxidation of Ti3C2Tx nanosheets at high temperatures (up to 600 oC). The synthesized OMCTs have high specific surface areas, high thermal and mechanical stability, and ordered mesoporous structures. The obtained 2D ordered mesoporous carbon/MXene heterostructure effectively inhibited the aggregation and oxidation of MXene and improved its electrochemical performance.
Graphene is an excellent conductive material widely used as a substrate for synthesizing 2DMMs[48,63,64]. Wang et al. prepared mesoporous nanosheets of polyaniline decorated on graphene (PANI/G) using a 2D template method. Specifically, PS100-PEO115 micelles were adsorbed on the surface of GO nanosheets by electrostatic adsorption and hydrogen bond force to obtain PS-PEO@GO[65]. Then, ammonium persulfate was used as an initiator to initiate the polymerization of aniline on the PS-PEO@GO surface to obtain PANI/PS-PEO@GO. Subsequently, the PS-PEO template was removed by washing with THF. Finally, ordered mesoporous PANI/G nanosheets were obtained by hydrothermal heat treatment. In particular, the obtained nanosheets have flat 2D structures, uniform pore structures, transverse sizes of 1-3 μm, and surface mesoporous about 18 nm.
According to the summary above, the multi-templates method is simple and versatile for preparing 2DMMs. Compared with other synthesis methods, the multi-templates method can effectively design and control the mesostructure and 2D morphology of the customized 2DMMs. However, it remains challenging to achieve narrow distributions and well-defined dimensions of mesoporous structures in 2DMMs by this method.
SURFACE-LIMITED CO-ASSEMBLY METHOD
The traditional method of synthesizing mesoporous materials using amphiphilic molecules as structure guide agents usually uses substrates such as glass, silicon wafers, and others for solid-liquid interface assembly synthesis. The obtained materials have low long-term continuity of pore channels and long mass transfer distance and are difficult to fully utilize the active sites in pores, which seriously hinders their application.
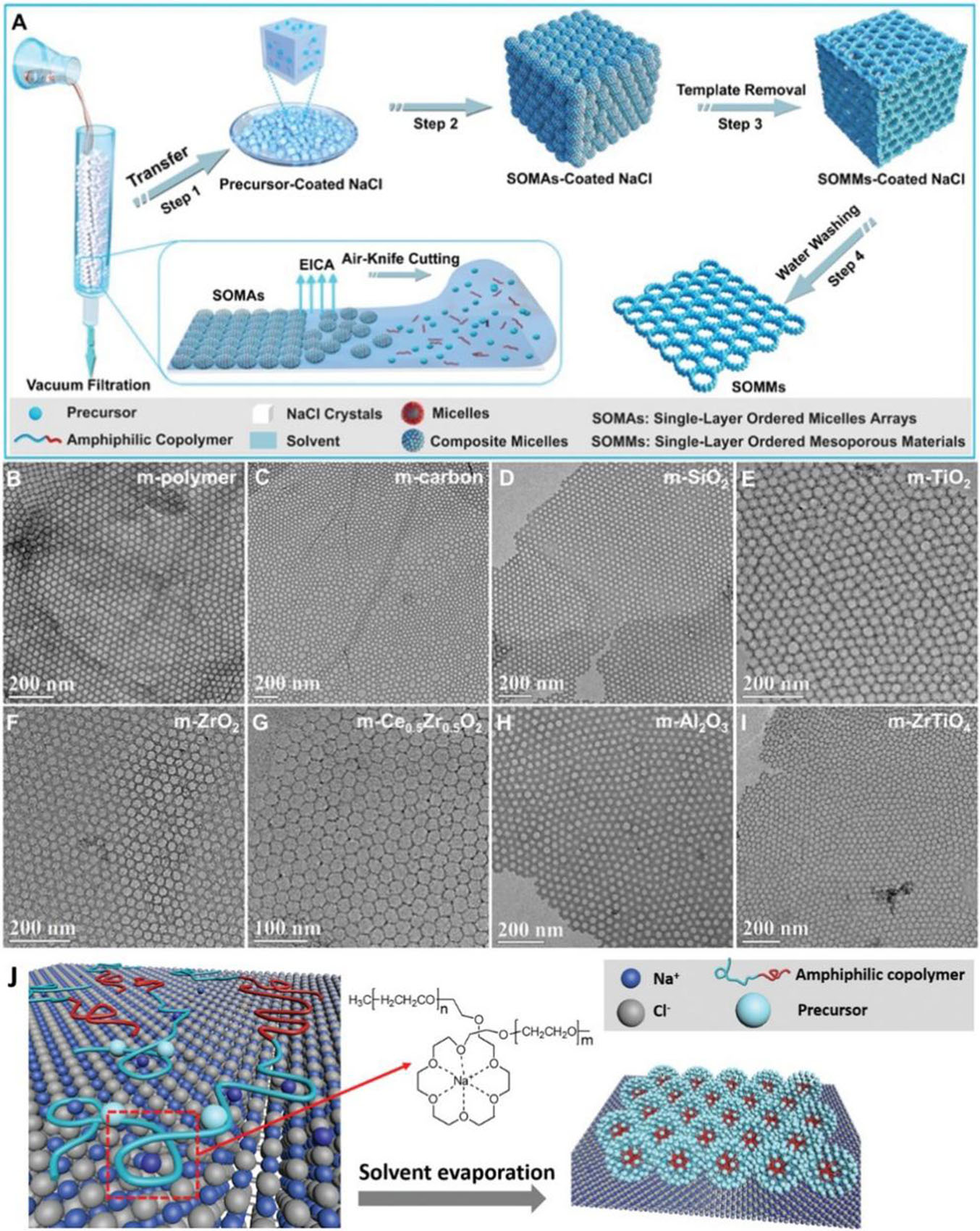
To solve the above dilemma, the “SLCA” strategy was proposed[66], which is a universal, controllable, and suitable method for the preparation of 2D single-layered ordered mesoporous materials on a large scale. The SLCA uses block copolymers and precursors to assemble on the surface of salt particles in order to form a single layer micelle bound to the surface of salt particles so as to obtain 2DMMs.
For the first time, the surface of inorganic salt (such as NaCl) particles was used as the substrate for assembly [Figure 7A]. Combined with the method of vacuum filtration to remove excess precursor liquid, the interaction between block copolymer guide agent and crown ether on the surface of salt particles and the synergistic co-assembly between them and precursor molecules/nanocrystals was utilized [Figure 7B]. It is noteworthy that the flexible polyether PEO segments of structural directing agents can play a role similar to crown ethers during the assembly process, complexing with cations (Na+, K+, etc.) on the surface of inorganic salt particles and generating strong interactions with the surface of inorganic salt particles, thereby ensuring that the block copolymers and precursors are orderly assembled on the surface of salt particles under synthetic conditions, forming a single layer of micelles bound by the surface of salt particles. The controllable synthesis of 2D mesoporous carbon, silica, and metal oxides (TiO2, CeO2, Al2O3, ZrO2,
Figure 7. (A) Schematic illustration of the fabrication process of the SLCA strategy; (B-I) TEM images of 2DMMs of different compositions: (B) m-polymer (phenolic formaldehyde resin); (C) m-carbon; (D) m-SiO2; (E) m-TiO2; (F) m-ZrO2; (G) m-Ce0.5Zr0.5O2; (H) m-Al2O3; and (I) m-ZrTiO4; (J) Schematic illustration of the interactions between block copolymers and the surface of NaCl salt crystals. This figure is quoted with permission from Liu et al.[66]. SLCA: Surface-limited co-assembly; TEM: transmission electron microscopy; 2DMMs: two-dimensional mesoporous materials.
Due to the diverse accessible surfactants and precursors, this SLCA method provides new possibilities for the designed synthesis of various kinds of 2DMMs[67], including the application of multi-component metal oxides, metal sulfides, and carbide nanosheets.
TEMPLATE-FREE METHOD
The template-free method is a simple and general method for preparing 2DMMs without the use of 2D and mesoporous structural guiding agents[68-71]. The 2D framework is spontaneously formed through self-assembly, ion/molecular intercalation, calcination, or gas induced detachment, together with mesoporous cavities generated by the precursor decomposition, gas release, structural defects, and nanoparticle aggregation.
For instance, Hu et al. reported a template-free synthesis strategy for the preparation of highly crystalline 2D mesoporous transition metal oxide nanosheets with abundant surface defects[72]. The method is based on the crystal-crystal transition to convert the highly crystallized basic carbonate 2D nanosheets into the highly crystallized transition metal oxide 2D nanosheets at low temperatures. During the thermal transition process, due to the removal of small molecules (carbon dioxides and water molecules), a double-through mesoporous structure is generated in situ, which results in highly crystallized 2D mesoporous transition metal oxides with regular morphology. The method is simple and universal and can realize the controllable preparation of a variety of high crystalline mesoporous transition metal oxides. Moreover, different from the conventional crystalline mesoporous metal oxides, the mesoporous transition metal oxides obtained by this method have abundant surface-step defects.
Recently, Kaneti et al. synthesized 2D mesoporous mixed oxide nanosheets by a template-free method[73]. The simple long-term reaction of metal glyceric acid nanospheres in water at room temperature resulted in the gradual decomposition of organic groups in the glyceric acid nanospheres, which were subsequently replaced by hydroxyl nanosheets and self-deconstructed/reconstructed into 2D Co glyceric acid/hydroxide nanosheets [Figure 8]. Two-dimensional cobaltate nanosheets obtained by this “self-deconstruction/reconstruction” strategy exhibit high surface areas and narrow pore size distributions.
Figure 8. Schematic illustration showing the proposed general template-free strategy for 2DMMs. This figure is quoted with permission from Kaneti et al.[73]. 2DMMs: Two-dimensional mesoporous materials.
Nevertheless, due to the mesopores originating from the pores between or within particles of the material, a template-free method generally lacks the ability to control the size and structure of pores and often can only obtain disordered mesopores.
CONCLUSION AND OUTLOOK
In this paper, the progress and achievements in the preparation of 2DMMs from self-assembly methods in the past ten years are reviewed. A self-assembled synthetic strategy provides flexible control over the morphology and pore size/shape of 2DMMs. The current popular synthesis methods are discussed, including molecular self-assembly methods, single micelle assembly methods, multi-templates methods, SLCA methods, and template-free methods. Through these methods, various 2DMMs with adjustable sizes and morphology have been achieved.
A molecular self-assembly method is a general method for the controlled synthesis of 2DMMs with ordered porous structures, which can realize the precise control of the composition, coordination environment, and nanostructure of the designed catalyst. The single micelle method can achieve the preparation of ultra-thin monolayer mesoporous structures by precise self-assembly at the level of a single micelle. The multi-templates method relies on the preparation of mesoporous inorganic/organic layers on 2D-oriented substrates and is suitable for the preparation of planar mesoporous nanosheets or sandwich mesoporous heterostructures. SLCA is suitable for preparing 2DMMs with highly open porous structures, short diffusion distances, and easy access to the active site. Template-free methods are often used to prepare disordered 2DMMs due to their lack of ability to control pore sizes and structures. The comparison of the currently available synthetic methods toward 2DMMs is as follows [Table 1].
Comparison of the currently available synthetic strategies toward 2DMMs
| Methods | Advantages | Disadvantages |
| Molecular self-assembly method | Controllable preparation; Easy removal of templates without residue | Strict reaction conditions |
| Single micelle assembly method | Precise synthesis control; Open pore structure | Poor crystallinity |
| Multi‑templates method | Well design and control the mesostructure and 2D morphology | Wide pore size distribution |
Surface-limited co-assembly method | Diverse accessible surfactants and precursors; Strong universality | Complex operation |
| Template‑free method | Simple and general | Disordered mesopores |
Compared to homologous 2D non-mesoporous materials and bulk mesoporous materials, the unique features of 2DMMs, such as larger lateral sizes, abundant active sites, thinner thickness, and more open pore channels, improve the performance of materials in applications such as energy storage, catalysis, and so on. In addition, 2DMMs with different structures can endow materials with different application prospects. For example, sandwich-like mesoporous heterostructures of medium-thickness 2DMMs can balance the content of functional components and 2D substrates and maximize their application properties. 2DMMs with vertical spherical pores typically have larger specific surface areas, thereby exhibiting higher specific capacitance and energy density in the electrochemical field. Disordered 2DMMs possess interconnected mesoporous and larger specific surface areas and thus enhanced mass/ion transfer efficiency, while ordered mesoporous can endow 2DMMs with controllable morphology, special functions, and adjustable application properties.
However, 2DMMs are still in their early stages, and there are the following development issues that need to be addressed urgently. Firstly, although there have been encouraging developments in the designed synthesis of 2DMMs, their chemical compositions and synthesis strategies still cannot meet the requirements. In order to obtain 2DMMs with accurate synthetic composition, geometric shape, and controllable pore sizes, it is urgent to explore simpler and more universal synthesis methods and deeply understand their formation mechanisms. Secondly, current research on 2DMMs mainly focuses on commonly used polymers (such as polypyrrole, polydopamine, and polydiaminopyridines), carbon (including graphene), oxides (such as SiO2 and TiO2), and their hybrids. It can be predicted that with the development of ordered mesoporous atomic sheets, new type 2DMMs (such as carbides, nitrides, sulfides, phosphides, selenide, and metal/covalent organic frameworks) with diverse single or multi-component components will be developed, the designed preparation of 2D mesoporous heterostructures and composites will open up an unknown new design space for material science. Thirdly, 2DMMs are expected to make breakthroughs in applications such as membrane separation, catalysis, energy storage, solar cells, electronic devices, etc. However, their manufacturing in the future still needs to expand in scale. Therefore, in order to meet the needs of industry or commercialization, it is also crucial to prepare high-quality 2DMMs on a large scale and at a low cost.
DECLARATIONS
Authors’ contributionsPrepared and revised the manuscript: Feng D
Revised the manuscript: Li X, Zhang L
Designed and revised the manuscript: Qiao ZA
All authors contributed to the discussion and preparation of the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China (22105033, 21621001, and 21671073), the Science and Technology Department of Jilin Province (YDZJ202201ZYTS330 and YDZJ202101ZYTS137), the “111” Project of the Ministry of Education of China (B17020), and Program for JLU Science and Technology Innovative Research Team, Interdisciplinary Integration and Innovation Project of Jilin University.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Perego C, Millini R. Porous materials in catalysis: challenges for mesoporous materials†. Chem Soc Rev 2013;42:3956-76.
2. Du G, Xu Y, Zheng S, Xue H, Pang H. The state of research regarding ordered mesoporous materials in batteries. Small 2019;15:e1804600.
3. Li C, Li Q, Kaneti YV, Hou D, Yamauchi Y, Mai Y. Self-assembly of block copolymers towards mesoporous materials for energy storage and conversion systems. Chem Soc Rev 2020;49:4681-736.
4. Li W, Liu J, Zhao D. Mesoporous materials for energy conversion and storage devices. Nat Rev Mater 2016;1:16023.
5. Zhuang Z, Li Y, Yu R, et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat Catal 2022;5:300-10.
6. Singh G, Lee J, Karakoti A, et al. Emerging trends in porous materials for CO2 capture and conversion. Chem Soc Rev 2020;49:4360-404.
7. Yang X, Deng Y, Yang H, et al. Functionalization of mesoporous semiconductor metal oxides for gas sensing: recent advances and emerging challenges. Adv Sci 2022;10:e2204810.
8. Sun L, Lv H, Feng J, et al. Noble-metal-based hollow mesoporous nanoparticles: synthesis strategies and applications. Adv Mater 2022;34:2201954.
9. Liu Z, Du Y, Yu R, et al. Tuning mass transport in electrocatalysis down to sub-5 nm through nanoscale grade separation. Angew Chem Int Ed 2023;62:e202212653.
10. Zhuang Z, Li Y, Li Y, et al. Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides†. Energy Environ Sci 2021;14:1016-28.
11. Ren Y, Ma Z, Bruce PG. Ordered mesoporous metal oxides: synthesis and applications. Chem Soc Rev 2012;41:4909-27.
12. Lin B, Yang G, Wang L. Stacking-layer-number dependence of water adsorption in 3D ordered close-packed g-C3N4 nanosphere arrays for photocatalytic hydrogen evolution. Angew Chem Int Ed 2019;58:4587.
13. Hwang J, Kim S, Wiesner U, Lee J. Generalized access to mesoporous inorganic particles and hollow spheres from multicomponent polymer blends. Adv Mater 2018;30:1801127.
14. Tan C, Cao X, Wu XJ, et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem Rev 2017;117:6225-331.
15. Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science 2004;306:666-9.
16. Jin H, Guo C, Liu X, et al. Emerging two-dimensional nanomaterials for electrocatalysis. Chem Rev 2018;118:6337-408.
17. Duan J, Chen S, Jaroniec M, Qiao SZ. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal 2015;5:5207-34.
18. Manzeli S, Ovchinnikov D, Pasquier D, Yazyev OV, Kis A. 2D transition metal dichalcogenides. Nat Rev Mater 2017;2:17033.
19. Zhou D, Li P, Lin X, et al. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem Soc Rev 2021;50:8790-817.
20. VahidMohammadi A, Rosen J, Gogotsi Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021;372:eabf1581.
21. Park J, Lee J, Kim S, Hwang J. Graphene-based two-dimensional mesoporous materials: synthesis and electrochemical energy storage applications. Materials 2021;14:2597.
22. Kim S, Lim WG, Im H, et al. Polymer interface-dependent morphological transition toward two-dimensional porous inorganic nanocoins as an ultrathin multifunctional layer for stable lithium-sulfur batteries. J Am Chem Soc 2021;143:15644-52.
23. Qin J, Yang Z, Xing F, Zhang L, Zhang H, Wu Z. Two-dimensional mesoporous materials for energy storage and conversion: current status, chemical synthesis and challenging perspectives. Electrochem Energy Rev 2023;6:9.
24. Kim S, Ju M, Lee J, Hwang J, Lee J. Polymer interfacial self-assembly guided two-dimensional engineering of hierarchically porous carbon nanosheets. J Am Chem Soc 2020;142:9250-7.
25. Kim S, Hwang J, Lee J, Lee J. Polymer blend directed anisotropic self-assembly toward mesoporous inorganic bowls and nanosheets. Sci Adv 2020;6:eabb3814.
27. Deng Y, Wei J, Sun Z, Zhao D. Large-pore ordered mesoporous materials templated from non-Pluronic amphiphilic block copolymers†. Chem Soc Rev 2013;42:4054-70.
28. Lakhi KS, Park DH, Al-Bahily K, et al. Mesoporous carbon nitrides: synthesis, functionalization, and applications. Chem Soc Rev 2017;46:72-101.
30. Zhang X, Hou L, Ciesielski A, Samorì P. 2D materials beyond graphene for high-performance energy storage applications. Adv Energy Mater 2016;6:1600671.
31. Duan L, Wang C, Zhang W, et al. Interfacial assembly and applications of functional mesoporous materials. Chem Rev 2021;121:14349-429.
32. Wang L, Urbas AM, Li Q. Nature-inspired emerging chiral liquid crystal nanostructures: from molecular self-assembly to DNA mesophase and nanocolloids. Adv Mater 2020;32:1801335.
33. Zhu H, Sun S, Hao J, et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis†. Energy Environ Sci 2023;16:619-28.
34. Zhuang Z, Xia L, Huang J, et al. Continuous modulation of electrocatalytic oxygen reduction activities of single-atom catalysts through p-n junction rectification. Angew Chem Int Ed 2023;62:e202212335.
35. Song Y, Norris F, Hinchcliffe D, Xu Y, Zhang X, Nockemann P. Ionic liquid-assisted synthesis of mesoporous polymers and carbon materials: the self-assembly mechanism†. Nanoscale 2022;14:14212-22.
36. Chen Y, Tian M, Liu X. Supramolecular self-assembly strategy towards fabricating mesoporous nitrogen-rich carbon for efficient electro-fenton degradation of persistent organic pollutants. Nanomaterials 2022;12:2821.
37. Xiao Y, Tian G, Li W, et al. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis. J Am Chem Soc 2019;141:2508-15.
38. Qiu P, Zhao T, Fang Y, et al. Pushing the limit of ordered mesoporous materials via 2D self-assembly for energy conversion and storage. Adv Funct Mater 2021;31:2007496.
39. Zu L, Qian X, Zhao S, et al. Self-assembly of ir-based nanosheets with ordered interlayer space for enhanced electrocatalytic water oxidation. J Am Chem Soc 2022;144:2208-17.
40. Zhang R, Liu Z, Gao TN, et al. A solvent-polarity-induced interface self-assembly strategy towards mesoporous triazine-based carbon materials. Angew Chem Int Ed 2021;60:24299.
41. Han G, Yang Y, Feng D, et al. Interface and charge induced molecular self-assembly strategy for the synthesis of reduced graphene oxide coated with mesoporous platinum sheets. Macromol Rapid Commun 2022;43:2100923.
42. Liu S, Wang F, Dong R, et al. Dual-template synthesis of 2D mesoporous polypyrrole nanosheets with controlled pore size. Adv Mater 2016;28:8365-70.
43. Wang F, Zhang Z, Shakir I, Yu C, Xu Y. 2D polymer nanosheets for membrane separation. Adv Sci 2022;9:2103814.
44. Tang N, Chen Y, Li Y, Yu B. 2D polymer nanonets: controllable constructions and functional applications. Macromol Rapid Commun 2022;43:2200250.
45. Kang J, Huang S, Jiang K, et al. 2D Porous polymers with sp2-carbon connections and sole sp2-carbon skeletons. Adv Funct Mater 2020;30:2000857.
46. Xue R, Zheng Y, Zhang L, et al. A novel 2D mesoporous phosphazene-anthraquinone-based covalent organic polymer: synthesis, characterization and supercapacitor applications†. New J Chem 2021;45:19125-31.
47. Zhang L, Liu Y, Wang T, Liu Z, Li W, Qiao ZA. Multi-dimensional molecular self-assembly strategy for the construction of two-dimensional mesoporous polydiaminopyridine and carbon materials. Small 2023;19:2205693.
48. Yang Y, Song X, Yao Y, et al. Ultrasmall single micelle@resin core-shell nanocarriers as efficient cargo loading vehicles for in vivo biomedical applications†. J Mater Chem B 2015;3:4671-8.
49. Zhao T, Elzatahry A, Li X, Zhao D. Single-micelle-directed synthesis of mesoporous materials. Nat Rev Mater 2019;4:775-91.
50. Zhou Q, Zhao T, Liu M, et al. Highly stable hybrid single-micelle: a universal nanocarrier for hydrophobic bioimaging agents. Nano Res 2022;15:4582-9.
51. Qiu P, Yang J, Jiang W, Wang L, Fan Y, Luo W. Interfacial engineering of core-shell structured mesoporous architectures from single-micelle building blocks. Nano Today 2020;35:100940.
52. Lan K, Wei Q, Wang R, et al. Two-dimensional mesoporous heterostructure delivering superior pseudocapacitive sodium storage via bottom-up monomicelle assembly. J Am Chem Soc 2019;141:16755-62.
53. Wang R, Lan K, Lin R, et al. Precisely controlled vertical alignment in mesostructured carbon thin films for efficient electrochemical sensing. ACS Nano 2021;15:7713-21.
54. Lan K, Liu L, Yu J, et al. Stepwise monomicelle assembly for highly ordered mesoporous TiO2 membranes with precisely tailored mesophase and porosity. JACS Au 2023;3:1141-50.
55. Xi X, Wu D, Han L, et al. Highly uniform carbon sheets with orientation-adjustable ordered mesopores. ACS Nano 2018;12:5436-44.
56. Li Y, Liu Y, Li J, et al. A centimeter scale self-standing two-dimensional ultra-thin mesoporous platinum nanosheet†. Mater Horiz 2020;7:489-94.
57. Liu S, Zhang J, Dong R, et al. Two-dimensional mesoscale-ordered conducting polymers. Angew Chem Int Ed 2016;55:12516.
58. Li X, Guan Q, Zhuang Z, et al. Ordered mesoporous carbon grafted MXene catalytic heterostructure as Li-Ion kinetic pump toward high-efficient sulfur/sulfide conversions for Li-S battery. ACS Nano 2023;17:1653-62.
59. Li Q, Xu X, Guo J, et al. Two-dimensional MXene-polymer heterostructure with ordered in-plane mesochannels for high-performance capacitive deionization. Angew Chem Int Ed 2021;60:26528.
60. Shi Y, Li B, Zhu Q, et al. MXene-based mesoporous nanosheets toward superior lithium ion conductors. Adv Energy Mater 2020;10:1903534.
61. Fang Y, Lv Y, Gong F, Elzatahry AA, Zheng G, Zhao D. Synthesis of 2D-mesoporous-carbon/MoS2 heterostructures with well-defined interfaces for high-performance lithium-ion batteries. Adv Mater 2016;28:9385-90.
62. Liu Z, Xiong H, Luo Y, et al. Interface-induced self-assembly strategy toward 2D ordered mesoporous carbon/MXene heterostructures for high-performance supercapacitors. ChemSusChem 2021;14:4422.
63. Yang S, Feng X, Müllen K. Sandwich-like, graphene-based titania nanosheets with high surface area for fast lithium storage. Adv Mater 2011;23:3575-9.
64. Yang S, Yue W, Zhu J, Ren Y, Yang X. Graphene-based mesoporous SnO2 with enhanced electrochemical performance for lithium-ion batteries. Adv Funct Mater 2013;23:3570-6.
65. Wang X, Qin J, Hu Q, et al. Multifunctional mesoporous polyaniline/graphene nanosheets for flexible planar integrated microsystem of zinc ion microbattery and gas sensor. Small 2022;18:2200678.
66. Liu L, Yang X, Xie Y, et al. A universal lab-on-salt-particle approach to 2D single-layer ordered mesoporous materials. Adv Mater 2020;32:1906653.
67. Yang X, Li Y, Ma J, et al. General and efficient synthesis of two-dimensional monolayer mesoporous materials with diverse framework compositions. ACS Appl Mater Interfaces 2021;13:1222-33.
68. Wang D, Shan Z, Tian J, Chen Z. Understanding the formation of ultrathin mesoporous Li4Ti5O12 nanosheets and their application in high-rate, long-life lithium-ion anodes†. Nanoscale 2019;11:520-31.
69. Fuertes AB, Sevilla M. Hierarchical microporous/mesoporous carbon nanosheets for high-performance supercapacitors. ACS Appl Mater Interfaces 2015;7:4344-53.
70. Liu S, Xu J, Li X, et al. Template-free self-assembly of two-dimensional polymers into nano/microstructured materials. Molecules 2021;26:3310.
71. Heydarian H, Schueder F, Strauss MT, et al. Template-free 2D particle fusion in localization microscopy. Nat Methods 2018;15:781-4.
72. Hu M, Yang W, Tan H, et al. Template-free synthesis of mesoporous and crystalline transition metal oxide nanoplates with abundant surface defects. Matter 2020;2:1244-59.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Feng D, Li X, Zhang L, Qiao ZA. Self-assembly method for two-dimensional mesoporous materials: a review for recent progress. Chem Synth 2023;3:37. http://dx.doi.org/10.20517/cs.2023.26
AMA Style
Feng D, Li X, Zhang L, Qiao ZA. Self-assembly method for two-dimensional mesoporous materials: a review for recent progress. Chemical Synthesis. 2023; 3(4): 37. http://dx.doi.org/10.20517/cs.2023.26
Chicago/Turabian Style
Feng, Danyang, Xuefeng Li, Ling Zhang, Zhen-An Qiao. 2023. "Self-assembly method for two-dimensional mesoporous materials: a review for recent progress" Chemical Synthesis. 3, no.4: 37. http://dx.doi.org/10.20517/cs.2023.26
ACS Style
Feng, D.; Li X.; Zhang L.; Qiao Z.A. Self-assembly method for two-dimensional mesoporous materials: a review for recent progress. Chem. Synth. 2023, 3, 37. http://dx.doi.org/10.20517/cs.2023.26
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 8 clicks
Cite This Article 8 clicks

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.