Recent advances in organocatalytic cascade reactions for enantioselective synthesis of chiral spirolactone skeletons
Abstract
Chiral spirolactones, including spiropropyllactones, spirobutyrolactones, and spirovalerolactones, are important heterocyclic frameworks that attracted the attention of organic and medicinal chemists because these motifs constitute the core structure of several natural products and bioactive molecules. The absolute configuration and the substituents on the fully substituted spirocyclic stereocenter of the lactone can potentially enhance specificity for ligand-protein binding and enhance bioavailability, potency, and metabolic stability. So, intensive attention from chemists has been paid to the synthetic methods leading to such prominent structural motifs. The synthetic methods can be divided into two main classes. The first approach takes advantage of the presence of the existing lactone structure and focuses on its functionalization. The second approach is the lactone framework constructed from various precursors in a direct spirolactonization reaction. In this review, for convenience in reading, the recent advances in the synthesis of spirolactones are summarized and discussed according to the two major organocatalytic asymmetric synthetic routes: (i) using the lactone-related frameworks as building blocks; and (ii) direct spirolactonization reaction using various reagents. This review also describes both the mechanisms and related transformations, and gives some insights into challenging issues in this research field, which will enlighten the future development of this field.
Keywords
INTRODUCTION
The biological activities of privileged natural products are usually linked with their characteristic moiety and well-defined stereo-structure[1,2]. This observation has provided the impetus to develop highly stereoselective synthetic strategies for the privileged target structure. Spirolactones, including spiropropyllactones, spirobutyrolactones and spirovalerolactones, are the structural motifs frequently found in many natural products and biologically active molecules [Figure 1][3-5]. The essential moiety of these compounds is the spiro-lactone core with various degrees of substitution. Due to their biological activities, these compounds have drawn much attention from chemists and biologists. However, many challenges need to be addressed, e.g., (a) control of stereogenic spirocenter; (b) incorporation of suitable functional groups into the newly formed ring system for the possible late-stage chemical modification and derivatization. Although several reports have been published in the past few years, the developing efficient methods to access chiral spirolactones with high structural diversity from readily available starting materials remain a challenging but highly desirable goal.
Figure 1. Typical biologically active spirolactones containing spiropropyllactones, spirobutyrolactones and spirovalerolactones.
The environmentally friendly, metal-free organocatalysis, usually under simple and mild reaction conditions, has been the frontier topic due to the described advantages[6-15]. In particular, organocatalytic asymmetric synthesis was awarded the 2021 Nobel Prize in Chemistry, which has been demonstrated as one of the most efficient methods for synthesizing chiral compounds. Thus, novel strategies for the organocatalytic asymmetric synthesis of chiral spirolactones are urgently needed. To address the aforementioned challenges, numerous elegant transformations have been developed for the organocatalytic asymmetric construction of this prominent structural motif, which usually employs the two major organocatalytic asymmetric synthetic approaches: the first approach takes advantage of the presence of the existing lactone structure and focuses on its functionalization; the second approach is the lactone framework constructed from various precursors in a direct spirolactonization reaction.
Despite ongoing progress, to the best of our knowledge, a comprehensive review article is lacking to summarize the recent advances in catalytic asymmetric synthesis of chiral spirolactones. Herein, we review for the first time the recent advances in organocatalytic asymmetric cascade reactions for synthesis of chiral spirolactone skeletons, including spirobutyrolactones and spirovalerolactones. This review is summarized and classified according to the two major organocatalytic asymmetric synthetic routes: (i) using the lactone-related frameworks as building blocks; and (ii) direct spirolactonization reaction using various reagents. Discussions of the asymmetric catalytic mechanisms and related transformations are also described. Finally, the remaining challenges in organocatalytic asymmetric synthesis of chiral spirolactones are also touched on, which will enlighten the future development of this research area.
USING THE LACTONE-RELATED FRAMEWORKS AS BUILDING BLOCKS IN ORGANOCATALYTIC ASYMMETRIC CASCADE REACTIONS
The novel skeleton could be obtained by the chemical recombination[16-20] of stereo-chemically rich scaffolds from different sources via complexity-generating transformations, which might exhibit unexpected or new bioactivities. Thus, the construction of these three-dimensional chiral spirolactone skeletons is highly desired in the pharmaceutical and organic synthetic community. The use of readily available lactone-related frameworks as building blocks in organocatalytic asymmetric cascade reactions is promising, which enables the construction of this type of potentially bioactive compound in one step [Scheme 1].
Scheme 1. Lactone-related frameworks as building blocks for the construction of chiral spirolactones.
Benzolactone-derived olefins as 2C synthons
The spiro benzolactone motif is a privileged synthetic moiety that can be found in many natural products with a wide range of biological activities [Figure 1]. For example, Rosmadial[21-23], isolated from aerial parts of Salvia pachyphylla, inhibits the growth of human cancer cells. Bis-diterpene ferrubietolide, which was isolated from one type of mahogany-dysoxylum lenticellare, was used as the pesticide. Abiesinol E, a member of the spiro-biflavonoid family, has antitumor-initiating effects[21-23]. It is a challenging task to perform asymmetric synthesis on a highly substituted spirocyclic ring, especially the quaternary chiral carbon.
Due to the well-known β-electrophilicity of benzolactone-derived olefins, they have become elegant spirobenzolactone scaffolds for organocatalytic asymmetric cascade annulation strategies. Nucleophilic addition of β-electrophilicity of benzolactone-derived olefins with electron-rich Michael acceptor via an intramolecular cycloaddition gave the spirolactone annulation product in one shot [Scheme 2]. Many different electron-rich substrates have been explored over the past few years.
In the past few years, several chiral phosphines catalyzed transformations have been noted[24-31], including the asymmetric synthesis of complicated chiral spirocyclic complex structures. In this context, in 2013, Albertshofer et al.[32] reported an efficient organocatalytic asymmetric [3 + 2] cycloaddition reaction between benzolactone-derived olefins 1 and a phosphonium ylide, derived from diverse Morita-Baylis-Hillman carbonates 2 under chiral phosphine catalysis to provide highly complex chiral spirocyclopentenebenzolactone scaffolds 3 with excellent enantioselectivity [Scheme 3]. Three continuous stereocenters, including one all-carbon spiro carbon center, were built in a single step. It is believed that the C2-symmetric phospholane C1 plays a critical role as an efficient nucleophilic catalyst for the described transformation.
Scheme 3. Asymmetric [3 + 2] cycloaddition reactions of benzolactone-derived olefins 1 and Morita-Baylis-Hillman carbonates 2. This figure is used with permission from the American Chemical Society[32].
Later on, Wang et al.[33] applied another organocatalytic asymmetric roadmap to diverse spirocyclopentenebenzolactones 5 and 7 catalyzed by a chiral phosphine C2 by employing benzolactone-derived olefins 1 and two types of allenic esters 4 and 6. In these reactions, γ-addition products 5 and α-addition products 7 can be obtained in high yields and with excellent enantioselectivities, respectively [Scheme 4].
Scheme 4. Regioselective catalytic asymmetric [3 + 2] cycloaddition of benzolactone-derived olefins 1 and two types of allenic esters 4 and 6. This figure is used with permission from the Royal Society of Chemistry[33].
The reaction started from the formation of a zwitterionic intermediate derived from the chiral phosphine and allenoate (4 or 6). Intermediate acted as a 1,3-dipole and underwent a [3 + 2] cycloaddition with benzofuranone 1 to give a product 5 via γ-addition or product 7 via α-addition. The DFT calculations disclosed the origins of the regioselective outcomes for this phosphine-catalyzed [3 + 2] cycloaddition reaction.
The tetrahydroquinoline scaffolds[34-36] can usually be found in natural products and drug molecules. Many of them have been used as therapeutic agents due to their biological activities. In the last few years, the application of squaramide organocatalysts in asymmetric catalysis resulted in the significant development of stereoselective cascade cycloaddition reactions.
In this context, in 2022, Zhang et al.[37] developed an asymmetric catalytic cascade reaction of benzolactone-derived olefins 1 and α, β-unsaturated ketones 9, merging the spiro-tetrahydroquinoline with spirobenzolactone into a single new skeleton through asymmetric catalytic cascade reactions catalyzed by a quinine-derived chiral bifunctional squaramide organocatalyst C3. A series of differently substituted spiro[benzolactone-tetrahydroquinoline] hybrids 9 was smoothly obtained with high yields and excellent diastereoselectivities and enantioselectivities (up to 99% yield, > 20:1 dr, and > 99% ee) under mild reaction conditions.
In the proposed reaction mechanism, initially, the bifunctional squaramide catalyst C3 synergistically activates both 1 and 8 through hydrogen bonds to form the reactive intermediate, followed by an aza-Michael addition process and an intramolecular cyclization, resulting in the final spiro product 9, accompanied by the regeneration of the chiral catalyst.
Further experiments demonstrated that the reaction of 9a with m-CPBA gave oxidation product 10a in 95% without damage of the stereo centers (> 20:1 dr and 95% ee, Scheme 5).
Scheme 5. Asymmetric catalytic cascade reactions of benzolactone-derived olefins 1 and α, β-unsaturated ketones 9. This figure is used with permission from the Royal Society of Chemistry[37].
Due to the significant medicinal value as well as the structural complexities of spiroheterocyclic hexahydroxanthones, the combination of two or more known pharmacophores could be an efficient and powerful method for the synthesis of bioactive natural product-based hybrid molecules[38-43]. Reaction of 1 with bifunctional pyrazolone-chromone 11 in the presence of quinine-derived thiourea catalyst C4 yielded complicated bispiro[benzolactone-pyrazolone-hexahydroxanthone]s 12 at ambient temperature
Scheme 6. Organocatalytic tandem reaction of pyrazolone-chromone 4C synthons 11 with benzolactone-derived olefins 1. This figure is used with permission from the Royal Society of Chemistry[44].
It is believed that the reaction proceeded via a domino inter-/intramolecular double Michael cycloaddition reaction, and it serves as a successful strategy for the access of complicate bispiro[benzolactone-pyrazolone-hexahydroxanthone]s 12, which contains five continuous stereocenters with two quaternary spiro stereocenters. The bis-spiro products 12 were obtained in up to 87% yield with > 20:1 dr and > 99% ee.
In the proposed reaction mechanism, it was revealed that the double hydrogen bonding interaction between 1 and catalyst C4 played a vital role in controlling the stereoselectivity of this cyclization reaction.
The three-dimensional complexity and diversity of the privileged natural product frameworks could be easily improved by the introduction of contiguous stereocenters[45,46]. In 2020, the synthesis of complicated spiro [benzolactone-hexahydroxanthone] framework 14 was reported.
In situ activation of benzolactone-derived olefin 1 with nitromethane through a [2 + 1] Michael addition generated chiral bifunctional donor-donor 3C synthon I with the assistance of quinidine-derived thiourea catalyst C5, followed with Michael/Henry cycloaddition with 3-formyl chromone 13 via urea-tertiary activation in a ‘‘one-pot’’ fashion, led to structurally diverse spiro [benzolactone-hexahydroxanthone]s 14 with up to six contiguous stereogenic centers, including a quaternary one [Scheme 7][47].
Scheme 7. Organocatalyzed three-component tandem reaction of benzolactone-derived olefins 1. This figure is used with permission from the Royal Society of Chemistry[47].
It is believed that the enantioselectivity was determined by the first Michael addition. While the Michal addition of I to the chromone core via hydrogen bonds stabilized intermediate followed by an intramolecular Henry cycloaddition was believed to be the substrate/catalyst-controlled diastereoselective process. All the final products were successfully isolated and characterized in up to 76% yield with > 20:1 dr and 98% ee.
Recently, Li et al.[48] developed a highly enantioselective Diels-Alder reaction of benzolactone-derived olefins 1 with polyenals 15 catalyzed by diphenylprolinolsilyl ether C6 through a trienamine activation strategy, which enabled diversity-oriented synthesis of a series of potentially bioactive multifunctional chiral spirocyclohexenebenzolactone scaffolds 16 with moderate diastereoselectivities and good enantioselectivities. It was demonstrated that this activation strategy lies within the perfect chirality relay over a distance of up to eight bonds by combining trienamine catalysis with enamine activation[Scheme 8A].
Scheme 8. Organocatalytic Diels-Alder reaction of benzolactone-derived olefins 1 and polyenals 15. This figure is used with permission from the American Chemical Society[48].
To further check the generality of the described work, late-stage chemical modification of the Diels-Alder adduct 16a was examined. As shown in Scheme 8B, adduct 16a could be efficiently converted to highly complex scaffolds 17a and 18a with good efficiency [Scheme 8B].
Similarly, asymmetric Diels-Alder reaction of 2,4,6-trienals via tetraenamine catalysis was also reported by Zhou et al.[49]. However, only one example was reported, which utilized benzolactone-derived olefins as a 2C synthon to afford the chiral spirocyclohexenebenzolactone complex in 96% ee and 10:1 dr.
In 2011, Cassani et al.[50] developed an organocascade double Michael addition strategy based on the enamine-iminium activation of α, β-unsaturated ketones 19. The highly modified cyclic spiro product 20 was obtained in excellent yields (up to 91%) and enantioselectivity (> 19:1 dr, > 99% ee) in one single chemical step along with the construction of two new bonds and three stereogenic centers [Scheme 9A].
Scheme 9. Organocascade double Michael addition of benzolactone-derived olefins 1 and α, β-unsaturated ketones 19. This figure is used with permission from the Royal Society of Chemistry[50].
This novel strategy demonstrated the unique ability of primary amine catalyst C7, which facilitates the formation of nucleophilic dienamine intermediate and controls the stepwise double-Michael addition. Thus, a variety of complicated spiro-products 20 was successfully isolated with a good diastereomeric and enantiomeric ratio [Scheme 9B].
As a continuation of the above-mentioned strategy, a three-component domino reaction was reported and the spirocyclic benzolactone compounds 23 was obtained by the sequential enamine-iminium-enamine activation of aldehydes 21 and α, β-unsaturated aldehydes 22 followed with dehydration as the final step
Scheme 10. Organocascade three-component domino reaction of benzolactone-derived olefins 1, aldehydes 21 and α, β-unsaturated aldehydes 22. This figure is used with permission from the Royal Society of Chemistry[50].
In addition, Chatterjee et al.[51] also extended the vinylogous triple cascade reaction of benzolactone-derived olefin. Thus, the three-component domino reaction proceeded by sequential Michael/1,6-addition/vinylogous aldol to give a sole product of spirocyclic benzolactone with six stereogenic centers with perfect control over the stereochemistry.
By combining cycloisomerization with trienamine catalysis, in 2016, Chintalapudi et al.[52] reported the enantioselective trienamine-organocatalyzed cycloadditions of pyrrolidinyl dienals, prepared by palladium-catalyzed cycloisomerization, successfully delivered two spiro[benzolactone-hexahydroindole] cycloadducts in high enantioselectivity with no preference of endo/exo selectivity.
The development of an efficient and straightforward methodology and enhancement of the structural diversity of these sulfur-containing 3,3’-pyrrolidonyl spirobenzolactones may have a promising impact on the physicochemical and biological characteristics of target molecules.
In this context, an efficient organocatalyzed asymmetric sequential Michael/cyclization of α-isothiocyanato imide 24 with benzothiolactone 1 under mild conditions was reported by Cao et al.[53]. However, only two sulfur-containing 3,3’-pyrrolidonyl spirobenzolactones 25 and 26 were reported[53]. Notably, when the oxygen atom of benzolactone-derived olefin 1 was replaced by sulfur, the enantioselective Michael/Cyclization reaction of thiobenzolactone-derived olefin 1 with α-isothiocyanato imide 24 also proceeded smoothly, which provided the spirothiobenzolactone skeleton 26 with greater than 99% ee and moderate distereoselectivity [Scheme 11].
Scheme 11. Organocascade Michael/Cyclization reaction of benzolactone-derived olefin 1 with α-isothiocyanato imide 24. This figure is used with permission from John Wiley and Sons Ltd[53].
α, β-Unsaturated butyrolactones/valerolactones as 2C synthons
The scaffold-inspired compounds encouraged us further to build a library of molecular complexity around the biologically relevant framework for further biological evaluation. The prevalent spiro butyrolactone/valerolactone moieties are present in many biologically active molecules and natural products, as shown in Figure 1. To access this type of potentially bioactive compounds, the use of readily available α, β-unsaturated butyrolactones/valerolactones 27 as dipolarophile synthons presents a promising one [Scheme 12]. However, due to the inherent inertness of the α, β-unsaturated esters, the corresponding report is still limited.
Scheme 12. α, β-unsaturated butyrolactones/valerolactones 27 as 2C synthons for construction of spirobutyrolactone/valerolactones.
Due to the inherent inertness of the α, β-unsaturated esters, there is no literature report regarding the catalytic asymmetric synthesis. Hence, it is critical to develop a novel organocatalytic system for it. In 2022, our group[54] established a highly efficient strategy for the synthesis of complicated bispiro[butyrolactone-pyrrolidine-indanedione] hybrids 30 in up to 89% yield with excellent selectivity (> 20:1 dr) with three-component reaction of α, β-unsaturated butyrolactones/valerolactones 27, ninhydrin 28 and proline or thioproline 29 [Scheme 13].
Scheme 13. Domino 1,3-dipolar cycloaddition of butyrolactones/valerolactones 27, ninhydrin 28 and proline or thioproline 29. This figure is used with permission from the Royal Society of Chemistry[54].
Initially, the intermediate azomethine ylides A was generated in situ by the reaction of ninhydrin 28 and proline or thioproline 29, followed by the cyclization addition of α, β-unsaturated butyrolactones/valerolactones 27 to obtain the final spiro product 30.
It is happy to find out that many of the natural products, e.g., parthenolide, dehydrocostus lactone, and costunolide, could be used as the substrates for described cyclization to give corresponding chiral parthenolide-fused product 30f, dehydrocostus lactone-fused products 30g-30h, and costunolide-fused product 30i with good yields (73%-81%) and enantioselectivity (> 20:1 dr).
The preliminary anti-cancer activity of three selected compounds 30f-h was performed [Scheme 14]. On the basis of the initial biological experiments results, 30f and 30g show significant cytotoxicity to the A549 cells and the K562 cells by inducing apoptosis of tumor cells. Thus, further optimization of the structure and biological data might make them potential candidates for drug discovery.
Scheme 14. The biological evaluation. This figure is used with permission from the Royal Society of Chemistry[54].
3-Alkenyl-5-arylbutenolides as 2C synthons
Due to the significant steric hindrance between the two vicinal spiro-quaternary chiral centers, especially in a constrained ring system, it is a great challenge to chemically synthesize such moiety. Hence, it is important to develop an efficient method for the synthesis of vicinal spiro-quaternary chiral centers.
Recently, 3-alkenyl-5-arylbutenolides serving as efficient 2C synthons have been used to generate highly functionalized spiro heterocyclic derivatives under organocatalytic conditions. Wang et al.[55] have developed a highly diastereo- and enantioselective [3 + 2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines 32 as efficient azomethine ylide precursors with 3-alkenyl-5-arylbutenolides 31 as efficient 2C synthons with thiourea-tertiary amine C10 as the catalyst (1 mol%) [Scheme 15].
Scheme 15. Organocascade 1,3-dipolar cycloaddition of 3-alkenyl-5-arylbutenolides 31 and N-2,2,2-trifluoroethylisatin ketimines 32. This figure is used with permission from the Royal Society of Chemistry[55].
In this reaction, the tertiary amine of the catalyst C10 activates the N-2,2,2-trifluoroethylisatin ketimines 32 via hydrogen bonding and simultaneously the 3-alkenyl-5-arylbutenolides 31 gets activated by thiourea group through hydrogen bonding. This process allows the rapid synthesis of spiro[pyrrolidin-3,20-oxindoles] 33 bearing four consecutive stereocenters, including sterically congested two vicinal quaternary spiro chiral centers, with excellent results (up to > 99% yield, > 20:1 dr, and > 99% ee).
The synthetic application of this cascade [3 + 2] cycloaddition was further demonstrated by a diversifying conversion of 33a into another two functionalized dispirocyclic heterocyclic derivatives 34 and 35 without damage to the stereocenters [Scheme 16].
Scheme 16. Representative transformations of product 33a. This figure is used with permission from the Royal Society of Chemistry[55].
Benzolactones as 1C synthons
The great synthetic challenge needs to be addressed for the stereo-controlled synthesis of highly functionalized polycyclic spirobenzolactone scaffolds, especially for the efficient installation of novel quaternary spiro stereocenters[56-59]. To overcome the synthetic challenge, benzolactones 36 containing a dinucleophilic center, have been utilized as highly efficient 1C precursors in combination with electron-deficient species as electrophiles in organocatalytic annulation systems for the synthesis of complex polycyclic spirobenzolactones [Scheme 17]. A variety of dielectrophiles have been explored over the past years.
In 2011, Chatterjee et al.[51] developed a novel amine-catalyzed cascade reaction of benzofuranones 36 with two molecules of enals 37 to access densely functionalized spirobenzolactone molecules 38 [Scheme 18]. The successful isolation and characterization demonstrated the application of organocascade for the synthesis of high degrees of stereochemical and architectural complexity in a single chemical transformation.
Scheme 18. Aminocatalytic cascade reaction of enals 37 with benzofuranones 36. This figure is used with permission from Wiley-VCH Verlag[51].
The plausible mechanism for organocascade was proposed. It is initiated with an iminium-catalysed Michael addition of 36 to 37 in the presence of C8 to give intermediate I. A second iminium-mediated Michael addition of the chiral nucleophilic intermediate I to another molecule of enal 37 is expected to give intermediate II. Lastly, an enamine-driven intramolecular aldol reaction of II followed by dehydration led to the formation of spirocyclic benzolactone 38 with excellent stereoselectivities [Scheme 18].
In addition, in 2010, Companyó et al.[60] developed an aminocatalytic Michael-Michael-aldol reaction, affording spirocyclic derivatives in good yields and in almost diastereo- and enantiopure form. However, only one example was reported that utilized benzolactone as a 1C synthon to afford the chiral spiro benzolactone compound in 93% ee and > 25:1 dr.
As a complementary above-mentioned organocascade strategy, in 2013, Li et al.[61] reported a highly enantioselective double-Michael addition reaction by reaction of benzofuranones 36 containing a dinucleophilic center with divinyl ketones 39 in the presence of tertiary amine-thiourea Cinchona alkaloid organocatalyst C11 for the construction of a spirobenzolactone scaffolds 40 in very good yields (up to 99%), up to 19:1 dr and 92% ee [Scheme 19A]. A broad substrate scope of spirobenzolactones 40 was obtained.
Scheme 19. Organocatalytic enantioselective double-Michael addition reaction of benzofuranones 36 and divinyl ketones 39. This figure is used with permission from John Wiley and Sons Ltd[61].
It was proposed that the tertiary amine of the catalyst C11 activates the divinyl ketone 39 via hydrogen bonding and simultaneously the benzofuranone 36 gets activated by thiourea through hydrogen bonding. The hydrogen bond interaction between the three components locked their conformation and played a critical role in the stereoselectivity of this cyclization reaction. The origin of stereoselectivity was explored and determined by density functional theory (DFT) calculations [Scheme 19A].
To demonstrate the synthetic utility of this process, the enantiomerically enriched compound 40a obtained by this method can be easily converted into oxime derivative 41 in one step without any loss of enantiomeric excess [Scheme 19B], which is an essential structural motif in a great number of drug molecules[62,63].
Benzolactone-chromones as 4C synthons
In 2020, our group developed a novel benzolactone-chromone building block 42 and successfully applied it to the organocatalytic Mannich/cyclization reactions of acceptor/donor-based species to give the chiral spirolactone derivatives with excellent structural complexity and diversity, thus facilitating the search for new bioactive entities. After careful analysis of the property of the structure, it is believed that this strategy benefits from the initial intermolecular Michael reaction followed by another intramolecular Michael reaction. The counterbalancing of the lower electrophilicity of these unactivated chromones is critical to facilitate the expected cyclization reaction [Scheme 20].
Due to the special structure of hexahydroxanthone, which contains four or more contiguous stereocenters, it represents a prominent structural motif in many natural products and pharmacologically active compounds. In this context, in 2020, a quinine-derived thiourea C12-catalyzed inter-/intramolecular Michael cycloaddition reaction of bifunctional benzolactone-chromone synthon 42 with benzolactone-derived olefins 1 was reported by Zhang et al. [Scheme 21][64]. All the desired products 43, potentially useful in medicinal chemistry, were obtained in good yields (up to 80%) with high diastereoselectivities (up to
Scheme 21. Organocatalyzed tandem reaction of benzolactone-chromone 4C synthons 42 with benzolactone-derived olefins 1. This figure is used with permission from Elsevier[64].
Bifunctional benzolactone-chromone synthon 42 was well-tailored to first act as a nucleophilic donor and then to originate as a Michael acceptor; after the conjugate addition, an in situ generated intermediate able to continue the intramolecular Michael cycloaddition.
α-Keto lactones as C-O synthons
The spiro-bis-lactone is a crucial synthetic motif found in natural products and biologically active molecules [Figure 1][65-70]. Benzolactone-2-one derivatives are typical α-keto lactones, which can serve as a new kind of versatile C-O synthons to react with electron-rich species in annulation, and have attracted much attention in recent years. Treatment of benzolactone-2-ones 43 with NHC-catalyzed generation of homoenolate equivalents from enals 44 in the presence of a convenient catalyst provided a straightforward strategy for the synthesis of spiro-bis-lactones 45 [Scheme 22A][71].
Scheme 22. N-heterocyclic carbene-catalyzed annulation of benzolactone-2-ones 43 and enals 44. This figure is used with permission from the Royal Society of Chemistry[71].
It was proposed that the addition of NHC to enal 44 gave an intermediate I, followed by nucleophilic addition to the ketone-carbonyl group of benzolactone-2-one 43 to give the intermediate I, which was tautomerized to intermediate III. Lastly, intermediate III underwent intramolecular nucleophilic addition to the activated carboxyl surrogate to close the lactone ring and the regenerated catalyst C went to the next circle [Scheme 22B]. It is important to note that no literature report regarding the catalytic asymmetric synthesis of such a molecule was available. Thus, it should be a great research potential for the chiral NHC-catalyzed asymmetric reactions.
Furthermore, α-keto lactone 43 could also serve as a powerful building block in asymmetric cascade [3 + 2] cycloaddition of α-isothiocyanato imide 46 to give the chiral spirolactone derivative 47 with structural complexity and diversity [Scheme 23]. However, only one example was reported[72]. Thus, it should be a great research potential for the aldol/cyclization reaction of α-keto lactone 43.
Scheme 23. Organocatalyzed aldol/cyclization reaction of α-keto lactone 43 with α-isothiocyanato imide 46. This figure is used with permission from the American Chemical Society[72].
3-Isothiocyanato butyrolactones as C-N-C synthons
The structural complexity and richness in the stereogenic centers of spirocyclic compounds are generally important for their potentially useful pharmaceutical properties. Moreover, biological activity seems to be enhanced by replacing oxygen atoms in spirocyclic molecules with sulfur atoms. The isothiocyanates bearing an electron-withdrawing group at the α-position have proved to be particularly critical for the asymmetric synthesis of spirocyclic heterocyclic compounds[73-79]. Recently, 3-isothiocyanato butyrolactone 48 could also serve as a powerful C-N-C synthon in asymmetric [3 + 2] cycloaddition to give the chiral spirothiobenzolactone derivatives [Scheme 24].
Scheme 24. 3-Isothiocyanato butyrolactone as C-N-C synthons for construction of chiral spirobutyrolactones.
In 2010, a rosin-derived thiourea catalyst C9 catalyzed asymmetric aldol/cyclization cascade reaction of isothiocyanato butyrolactone 48 as C-N-C synthon with isatins 49 was reported by Jiang et al.[72]. It is proposed that, with the dual activation of rosin-based thiourea C9, this strategy allowed rapid construction of highly functional bispiro[thiooxazoline/thiocarbamate-butyrolactone-oxindole]s 50 and 51 with moderate diastereoselectivities (70:30-92:8 dr). However, the author did not provide their specific ees [Scheme 25].
Scheme 25. Organocatalyzed aldol/cyclization reaction of isothiocyanato butyrolactone 48 with isatins 49. This figure is used with permission from the American Chemical Society[72].
In addition, using α-isothiocyanato lactone and methyleneindolinone as the reactants, another organocatalytic synthesis of an optically active bispiro[oxindole-butyrolactone] scaffold was also reported by Cao et al.[53] The product having three contiguous stereocenters, including two spiro-quaternary chiral centers, was isolated with > 99% ee, > 20:1 dr and in 80% yield.
In 2011, employing isothiocyanato butyrolactone 48 as a C-N-C synthon, Jiang et al.[80] continued to report a highly efficient organocatalyzed Mannich/cyclization reaction of N-tosylimines 52 [Scheme 26].
Scheme 26. Organocatalyzed Mannich/cyclization reaction of isothiocyanato butyrolactone 48 with N-tosylimines 52. This figure is used with permission from Wiley-VCH Verlag[80].
In this reaction, the tertiary amine of the catalyst C18 activates the isothiocyanato butyrolactone 48 via hydrogen bonding and simultaneously the N-tosylimines 52 gets activated by thiourea group through hydrogen bonding. This process allows the rapid synthesis of optically active bispiro[thioimidazole-butyrolactone] skeletons 53 with high levels of enantio- and diastereoselectivity (up to 96% ee, and 10:1 dr).
Following the work of Jiang et al.[80], the reaction of isothiocyanato thiobutyrolactone 48 as a nucleophilic cascade with alkylidene pyrazolones 54 was explored for the squaramide-catalyzed enantioselective synthesis of bispirocyclic bispiro[pyrazolone-thiobutyrolactone] skeletons 55 via cascade Mannich/cyclization reaction. A range of structurally diverse products 55 bearing three contiguous stereocenters including two quaternary spiro stereocenters was obtained in up to 90% yield with up to > 20:1 dr and > 99% ee [Scheme 27A][81].
Scheme 27. Organocatalyzed Mannich/cyclization reaction of isothiocyanato thiobutyrolactone 48 with alkylidene pyrazolones 54. This figure is used with permission from the Royal Society of Chemistry[81].
As for the reaction mechanism, we speculated that the tertiary amine of the catalyst C16 activates the isothiocyanato thiobutyrolactone 48 via hydrogen bonding and simultaneously the alkylidene pyrazolones 54 gets activated by squaramide through hydrogen bonding. The hydrogen bond interaction between the three components locked their conformation and played a critical role in the stereoselectivity of this cyclization reaction [Scheme 27A]. To further highlight the synthetic value of this method, the amenability to gram-scale synthesis provided many opportunities for possible industrial applications [Scheme 27B].
To check the potential application of the demonstrated asymmetric synthesis, the reaction of 55e with MeI and K2CO3 in acetone led to the formation of methylated analog 56 without damage to the two quaternary spiro stereocenters [Scheme 27C]. Notably, there are two privileged substructures, butyrolactone and pyrazolone[82-87], in one spirocyclic molecular structure of 55, which may potentially be useful in medicinal chemistry.
Butyrolactone-derived cyclic imino esters as C-N-C synthons
The asymmetric 1,3-dipolar cycloaddition of azomethine ylides with electron-deficient olefins is one of the highly powerful methods for the synthesis of various highly substituted pyrrolidine scaffolds widespread in natural compounds. Recently, novel spirocyclic butyrolactone skeletons with multiple stereocenters could be constructed through simple 1,3-dipolar cycloadditions between butyrolactone-derived cyclic imino esters 57 as azomethine ylide precursors and electron-deficient species as dipolarophiles with suitable organocatalysts [Scheme 28].
Scheme 28. Butyrolactone-derived imino esters 57 as C-N-C synthons for construction of chiral spirobutyrolactones.
In 2013, a rosin-based thiourea C9 catalyzed formal 1,3-dipolar cycloaddition of butyrolactone-derived cyclic imino esters 57 and methyleneindolinones 58 as dipolarophiles was reported by Wang et al.
Scheme 29. Organocatalytic 1,3-dipolar cycloaddition of butyrolactone-derived imino esters 57 and methyleneindolinones 58. This figure is used with permission from the Royal Society of Chemistry[88].
In recent years, bispirocyclic scaffolds, which consist of three closed rings with two rigid spirocenters, have been found in many natural products that exhibit important biological activities. Asymmetric 1,3-dipolar cyclization reactions to construct these bispirocyclic scaffolds are highly attractive but extremely challenging. In this context, organocatalytic 1,3-dipolar cycloaddition of butyrolactone-derived cyclic imino esters 57 with alkylidene pyrazolones 60 in the presence of catalyst C17 in ether at ambient temperature gave highly functionalized bispiro products 61 in high yields (up to 93% yield) and excellent stereoselectivities (> 20:1 dr and > 99% ee, Scheme 30)[89]. Various highly functionalized bispiro[butyrolactone-pyrrolidin-pyrazolone] scaffolds 61 containing two quaternary spirocenters have been successfully obtained in a single reaction. Due to the extensive biological activities of the three structural motifs of butyrolactone, pyrrolidine, and pyrazolone, the author believes that the resulting bispiro[butyrolactone-pyrrolidin-pyrazolone] scaffolds 61 could potentially display enhanced or new bioactivities.
Scheme 30. Organocatalytic 1,3-dipolar cyclization of butyrolactone-derived cyclic imino esters 57 and alkylidene pyrazolones 60. This figure is used with permission from Wiley-VCH Verlag[89].
It was explained by the authors that both the intermolecular H-bonding between the catalyst and two substrates as well as the chiral environment created by the bifunctional squaramide catalyst C17 are critical for the generation of excellent stereoselectivities [Scheme 30].
Dihydrocoumarins and lactones are synthetically useful compounds with various bioactive derivatives and exist in many natural products. Both types of heterocyclic systems occupy a prominent position among the extraordinary richness of various heterocyclic systems. Recently, utilizing butyrolactone-derived cyclic imino esters 57 and α, β-unsaturated butenolides 62 as starting materials, Kowalczyk-Dworak et al.[90] have developed an organocatalytic approach for the preparation of spiro[butyrolactone-pyrrolidin-dihydrocoumarin] scaffolds 63 with structural complexity and diversity [Scheme 31].
Scheme 31. Organocatalytic annulation cascade reaction of α, β-unsaturated butenolides 62 and butyrolactone-derived cyclic imino esters 57. This figure is used with permission from the Royal Society of Chemistry[90].
There are two critical annulation processes in the reported synthesis. The first one is the [3 + 2]-dipolar cycloaddition for the construction of a pyrrolidine ring. The second one is the nucleophilic addition-elimination, which led to the butenolide-ring-opening and introduction of a dihydrocoumarin framework in 63. The polycyclic products 63 have been isolated and characterized in excellent yields and stereoselectivity due to the efficiencies of the bifunctional quinine-derived thiourea catalyst C18 [Scheme 31].
Heterocyclic fused chromanones have attracted lots of attention due to their structural complexity and significant medicinal activities. Inspired by the chemical and biological properties of butyrolactones, pyrrolidines as well as heterocycle-fused chromanones, we have successfully demonstrated the synthesis of novel spiro-[butyrolactone-pyrrolidine-chromanone] hybrids 65 by a simple domino 1,3-dipolar cycloaddition of azomethine ylides 57 with carboxylic acid-activated chromones 64 in the presence of the catalytic amount of Et3N [Scheme 32][91]. The desired products 65 were obtained in moderate to good yields (up to 85%) and selectivity (> 20:1 dr) via an exo-transition state. Although no literature report regarding catalytic asymmetric synthesis is available yet, further organocatalytic investigations should have great research potential.
Scheme 32. Organocatalytic domino 1,3-dipolar cycloaddition of butyrolactone-derived cyclic imino esters 57 and carboxylic acid-activated chromones 64. This figure is used with permission from Georg Thieme Verlag[91].
Furthermore, the biological activity of the four selected hybrids 65 was investigated [Scheme 33]. The experimental data shows impressive cytotoxicity to the K562 cells (IC50 < 50.00 μM) for 65a-65d. It is important to note that 65a and 65b also show considerable cytotoxicity to the A549 cells. Both of the promising biological data suggest the possible application of spiro-[butyrolactone-pyrrolidine-chromanone] derivatives 65 for the development of active drugs after further pharmacological studies.
Scheme 33. Evaluation of cytotoxicity toward cancer cell lines. This figure is used with permission from Georg Thieme Verlag[91].
In conclusion, the described works have demonstrated the potential of lactone-related frameworks as building blocks for the diversity-oriented synthesis of chiral spirolactone skeletons. Various complicate products could be easily obtained simply by taking advantage of the existing lactone structure and focusing on its functionalization. Thus, the efficient construction of chiral spirolactone-based libraries for modern probe and drug discovery will be another frontier in the near future.
DIRECT SPIROLACTONIZATION REACTION USING VARIOUS REAGENTS IN ORGANOCATALYTIC ASYMMETRIC CASCADE REACTIONS
The efficient construction of chiral spirolactones with broad structural diversity, including spiropropyllactones, spirobutyrolactones and spirovalerolactones from simple substrates, would be valuable for the development of new biologically active molecules.
To achieve this goal, in recent years, the use of readily available lactone-related frameworks as starting materials for the organocatalyzed asymmetric domino annulation reaction is a straightforward strategy. Despite the advancements, the structural diversity is limited by the choice of starting materials. In this context, the eco-friendly, efficient methodologies for the rapid generation of skeletally-diverse chiral spirolactone molecules remain highly desirable but challenging. In addition to utilizing the existing lactone structure as the starting material, another synthetic route is the skeletal construction of the lactone framework from various lactone construction precursors in a direct spirolactonization reaction, providing the structurally and biologically interesting chiral spirolactones with good efficiency [Scheme 34].
Spirolactonization reaction of 3-olefinic oxindoles
Skeletal construction is an important method in synthetic communities for the rapid and reliable construction of core frameworks with various functional groups. In 2018, Guo et al.[92] developed a new and promising Michael-initiated Pinnick oxidative spirolactonization reaction for the synthesis of potentially bioactive chiral spirocyclic oxindole-lactone derivatives 68 in up to 97% yield with up to 99% ee [Scheme 35, top]. The corresponding products 68 contain spirocyclic oxindole-paraconic esters and bear three chiral stereocenters.
Scheme 35. 3-Olefinic oxindoles as lactone construction reagents 66 in Michael-initiated Pinnick oxidative spirolactonization reactions. This figure is used with permission from the American Chemical Society[92].
The mechanism of formation of the spirocyclic oxindole-lactones is presented [Scheme 35, bottom]. Initially, an organocatalytic asymmetric Michael addition of aliphatic aldehyde 67 with 3-olefinic oxindole 66 to generate intermediate 3-oxindolepropionic aldehyde A. Then, spirocyclic oxindole-lactone 68 was generated in the presence of sodium chlorite via a sequential tandem Pinnick oxidation/chlorination/substitution transformation.
Spirolactonization reaction of 3-hydroxyoxindoles
The spiro[oxindole-lactone] scaffolds are found in a wide range of biologically active natural products and clinical pharmaceuticals [Figure 1]. Because of the correlation between molecular structure and biological activity, it is strongly desired to develop efficient asymmetric synthetic methods for the construction of spiro[oxindole-lactone] derivatives.
For many years, 3-hydroxyoxindoles 69 containing two reactive nucleophilic sites have been successfully applied in the organocatalytic asymmetric synthesis of spiro[oxindole-lactone] scaffolds. Due to the presence of the lone pair electron at the oxygen atom, they are a good candidate to react with electron-deficient species via nucleophilic addition reactions. With the presence of the carbonyl group, the sequential intramolecular nucleophilic lactonization reaction is expected via the acyl-transfer process [Scheme 36]. Various biselectrophilic species, such as α, β-unsaturated N-acylated succinimides, α, β-unsaturated pyrazoleamides, α, β-unsaturated esters, α, β-unsaturated acyl phosphonates and α, β-unsaturated acyl azoliums generated from enals and NHCs, have been explored over the past years.
Scheme 36. Spirolactonization reaction of 3-hydroxyoxindoles 69 for construction of chiral spirolactones.
The spirolactone is widely present in various natural products, e.g., fungi, plants, and marine species. Some of these products show significant biological and pharmaceutical activities [Figure 1][93,94]. To investigate the diversity-oriented synthesis of the medicinally important spirolactone scaffolds, an efficient tandem spirolactonization reaction of 3-hydroxyoxindoles 69 with olefinic azlactones 70 was reported by Cui et al.[95] for the synthesis of structurally diverse spirocyclic oxindole-lactones 71 [Scheme 37]. It is proceeded via the Michael addition of the 3-hydroxyoxindole 69 to the (Z)-olefinic azlactone 70 and sequential ring opening and lactonization.
Scheme 37. Spirolactonization reaction of 3-hydroxyoxindoles 69 and olefinic azlactones 70. This figure is used with permission from the American Chemical Society[95].
With DBU as the catalyst, various spirocyclic butyrolactone-oxindoles 71 were successfully isolated in high yields with excellent diastereoselectivities. However, no literature report regarding the catalytic asymmetric synthesis of spirocyclic oxindole-lactones 71 was found, which creates great research potential for asymmetric syntheses.
The α, β-unsaturated acyl phosphonates have been known as activated ester surrogates for the generation of esters or amides due to the presence of the lability of the C-P bond. In 2017, Chen et al.[96] developed a highly enantioselective Michael/lactonization cascade reaction of 3-hydroxyoxindoles 69 with α, β-unsaturated acyl phosphonates 72 in the presence of quaramide catalyst C19 under mild conditions (CAN, 25 °C). On the basis of the demonstrated protocol, a serial of spirocyclic oxindole-lactone derivatives 73 were obtained in moderate to excellent yields (up to 98%) with good to excellent diastereo- and enantioselectivities (up to > 99:1 dr and 95% ee) under the optimal conditions [Scheme 38].
Scheme 38. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated acyl phosphonates 72. This figure is used with permission from the American Chemical Society[96].
It is proposed that, with the dual activation of cinchonine-derived quaramide catalyst C19, Michael addition of the 3-hydroxyoxindoles 69 to α, β-unsaturated acyl phosphonates 72 forms the chiral acyl phosphonate intermediate B. Then, a favorable intramolecular cyclization reaction via the acyl-transfer led to the formation of spirocyclic oxindole-lactones 73 with excellent yields and selectivities [Scheme 38].
The class of heterocyclic spiro known as oxindole-lactones possesses many biological activities, e.g., tumor necrosis factor-α (TNFα) induced NF-κB inhibition, antibacterial, and antibiofilm activities[97,98]. Since trifluoromethylated molecules have unique physical, chemical, and physiological properties[99-101], it has been well developed in chemical biology and drug discovery. The structure-activity relationships (SAR) could be a good starting point for drug design. Hence the fusion of fluorinated lactones to oxindole will be discussed.
In this context, Yang et al.[102] developed an organocatalytic Michael/lactonization of 3-hydroxyoxindoles 69 with 3-trifluoroethylidene oxindoles 74 in the presence of a cinchona-derived squaramide catalyst C20 for the synthesis of heterocyclic spiro products 75Scheme 39). The conversion of the chemically inert amide moiety of oxindoles into the lactone was successfully achieved with the delivery of CF3-containing spiro oxindole-lactones 75 in moderate to excellent yields (up to 97%) and selectivities (up to 98:2 dr and 98% ee). It was explained by the authors that the dual activation of chiral bifunctional squaramide-tertiary amine catalyst C20 is critical for the generation of excellent stereoselectivities [Scheme 39].
Scheme 39. Spirolactonization reaction of 3-hydroxyoxindoles 69 and 3-trifluoroethylidene oxindoles 74. This figure is used with permission from the American Chemical Society[102].
In addition, the further late-stage modification of this asymmetric methodology was demonstrated by the treatment of 75a with HCl/MeOH at ambient temperature for 4 h. The 3-hydroxy oxindole 76 was obtained in 93% with 95% ee and 1.2:1 diastereoselectivity [Scheme 40].
Scheme 40. Chemical Transformation of Compound 75a. This figure is used with permission from the American Chemical Society[102].
Similar to α, β-unsaturated acyl phosphonates, the α, β-unsaturated N-acylated succinimides 77 can also serve as biselectrophilic species in the spirolactonization reaction of 3-hydroxyoxindoles 69. In 2017, Ming et al.[103] utilized α, β-unsaturated N-acylated succinimides 77 as biselectrophilic synthons to undergo a bifunctional squaramide-catalysed asymmetric Michael/cyclization cascade reaction of 3-hydroxyoxindoles 69, affording the desired spiro[oxindole-lactone] derivatives 78 bearing two contiguous stereocenters were obtained in up to 89% with up to > 95:5 dr and up to 99% ee [Scheme 41].
Scheme 41. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated N-acylated succinimides 77. This figure is used with permission from the Royal Society of Chemistry[103].
The plausible mechanism for the formation of 78 was proposed [Scheme 41]. Based on the activation of both substrates 69 and 77 with the bifunctional quinine-derived squaramide catalyst C21, the hydrogen bond stabilized intermediate A was obtained. The nucleophilic addition of C3 in the deprotonated 3-hydroxyoxindole 69 to the α, β-unsaturated N-acylated succinimide 77 via Michael addition gave intermediate B. Then, intramolecular lactonization was achieved by nucleophilic substitution of an alkoxide anion with the removal of the succinimide group to give the final product 78 [Scheme 41].
The α, β-unsaturated pyrazoleamides, as a class of excellent Michael receptors, have been widely used in asymmetric synthesis. Earlier in 2022, the Zn(OTf)2-catalyzed esterification of coumarin-3-pyrazoleamides 79 and 3-hydroxyoxindoles 69 in the presence of quinine-derived squaramide C22 for the synthesis of chiral spirocyclic oxindole-dihydrocoumarin-lactones 81 was reported by our group [Scheme 42][104]. It is proposed that, with the dual activation of quinine-derived squaramide C22, this cyclocondensation was realized by an esterification/asymmetric intramolecular Michael sequence. A serial of chiral spirocyclic product was obtained in excellent yields (up to 91% yield) and enantioselectivities (> 20:1 dr and up to 99% ee).
Scheme 42. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated coumarin-3-formylpyrazoles 79. This figure is used with permission from the American Chemical Society[104].
Further, to highlight the synthetic value of this method, we proceeded with a preparative-scale preparation of 81a under optimized reaction conditions [Scheme 43], and then obtained the desired product 81a in 88% yield, > 20:1 dr, 91% ee without deteriorating the stereochemical outcome of the reaction. The potential of the described asymmetric synthesis was further demonstrated by the late-stage modification via the ring-opening (DMA/THF, 75 °C, 30 min) or oxidation (DDQ/DCM, r.t. 5 h) to give other spiro-fused lactone derivatives 82 and 83, respectively [Scheme 43].
Scheme 43. Preparative-scale experiment and derivatizations. This figure is used with permission from the American Chemical Society[104].
The α-methylene-γ-lactone motif is one of the critical building blocks in organic synthesis. It has been found in a wide range of biologically active natural products and clinical pharmaceuticals[105-107]. It could also bind with specific proteins containing active SH groups from cysteine, which results in a change of the protein structure, thus inhibiting their functions[108-111]. So, the enantiopure hybrid structures combining α-methylene-γ-lactone motifs may pave the way to the generation of more promising covalent inhibitors for drug screening.
In this context, in 2020, the synthesis of highly functionalized spirooxindoles 85 was reported by Wang et al.[112] via the asymmetric allylic alkylation-cyclization of 3-hydroxyoxindoles 69 with MBH carbonates 84 in the presence of catalyst C23 in DMC at 5 °C. Due to the presence of α-methylene-γ-lactone decorated moiety in products 85, there is a possibility for the late-stage chemical modification after spirooxindoles 85 were obtained in good yields with diastereo- and enantioselectivities. It is proposed that nucleophilic addition (allylic alkylation) of 3-hydroxyoxindole 69 to MBH carbonates 84, which was activated by the cinchona alkaloid C23, followed by an intramolecular lactonization to afford the desired spirooxindoles 85 [Scheme 44A].
Scheme 44. Spirolactonization reaction of 3-hydroxyoxindoles 69 with MBH carbonates 84. This figure is used with permission from the Royal Society of Chemistry[112].
The potential of this methodology was demonstrated by the facile transformation for the construction of fused butenolide derivatives and their analogues. The cyclization product 85 was unexpectedly isomerized to the fused butenolide 86 in the presence of Pd-C/H2 in 41% yield. A CuCl-NaBH4 complex can successfully reduce 85 to the expected fused dihydrofuran-2(3H)-one derivative 87 in 38% yield and > 99:1 dr [Scheme 44B].
Another organocatalytic synthesis of optically active spirocyclic spirooxindole scaffolds 90 bearing α-methylene-γ-lactone motifs was reported by Jayakumar et al.[113]. Instead of 3-hydroxyoxindoles 69, 3-OBoc-protected oxindoles 88 were employed as a nucleophile, which avoided the possible dimerization of 3-hydroxyoxindole 69 under basic conditions as well as competitive C,O-alkylation. Furthermore, the alkylated product 90 was obtained under trifluoroacetic acid conditions, which is an important supplement to the above-mentioned Wang’s strategy [Scheme 45][112].
Scheme 45. Spirolactonization reaction of 3-OBoc-oxindoles 88 with MBH carbonates 89. This figure is used with permission from Wiley-VCH Verlag[113].
Recently, the N-heterocyclic carbene (NHC)-catalyzed reaction via radical intermediate has been well developed and used as a powerful strategy for the synthesis of highly modified molecules[114-116]. A variety of heterocycles including spiro compounds have been successfully synthesized in the presence of N-heterocyclic carbene (NHC) based organocatalysis. Besides the above-mentioned α, β-unsaturated substrates, the α, β-unsaturated aldehydes were also known as 3-carbon nucleophilic partners with 3-hydroxyoxindoles 69 for the synthesis of spiro compounds via the Michael addition/lactonization sequence.
In this context, in 2017, the N-heterocyclic carbene C25-catalyzed oxidative [3 + 2] annulation of 3-hydroxyoxindoles 69 and α, β-unsaturated aldehydes 91 was reported by Chen et al.[117] in 2017 [Scheme 46, top]. Both challenging alkyl enals and aryl enals 91 worked well under the optimal conditions to give the corresponding spirooxindole lactones 92 in good yields with excellent diastereo- and enantioselectivities.
Scheme 46. Spirolactonization reaction of 3-hydroxyoxindoles 69 with enals 91. This figure is used with permission from the Royal Society of Chemistry[117].
The cyclization was proposed to proceed via radical/radical cross-coupling of homoenolate and enolate, and the single electron transfer is the key step for NHC-catalyzed annulation reaction [Scheme 46, bottom]. Furthermore, both enolate and homoenolate radicals were observed by EPR spectra.
As a continuation of the research, the oxidative N-heterocyclic carbine C25-catalyzed [3 + 2] annulation of β, β-disubstituted enals 93 and 3hydroxyoxindole nucleophiles 69 was achieved via catalytic coupling of two tertiary radicals[118]. The spirocyclic oxindole-lactones 94, bearing two contiguous tetrasubstituted stereocenters, were obtained in good yields with excellent diastereoselectivities and good enantioselectivities. This is an important alternative synthetic method to the previously reported NHC catalysis strategy [Scheme 47][117].
Scheme 47. Spirolactonization reaction of 3-hydroxyoxindoles 69 with β, β-disubstituted enals 93. This figure is used with permission from the American Chemical Society[118].
The α, β-unsaturated acyl azolium intermediate[119,120] (electrophile) is an important intermediate in this N-heterocyclic carbene (NHC)-catalysis. This type of intermediate could be easily obtained from the reaction of enals and NHCs under oxidative conditions. Since they are good electrophiles, treatment with a variety of bisnucleophiles could give the corresponding heterocycles.
In this context, in 2017, the NHC-catalyzed enantioselective [3 + 2] annulation of α, β-unsaturated aldehydes 95 with 3-hydroxy oxindoles 69 in the presence of C26 gave spiro oxindole-butyrolactones 97 in moderate to good yields, enantioselectivity and diastereoselectivity [Scheme 48, top][121].
Scheme 48. Spirolactonization reaction of 3-hydroxyoxindoles 69 with enals 95. This figure is used with permission from the Royal Society of Chemistry[121].
Mechanistic investigation of this reaction revealed that the annulation was initiated with (1) the in situ generation of the chiral α, β-unsaturated acyl azolium intermediate or via (2) a formal [3 + 2] pathway to give the corresponding spirocyclic product 97 [Scheme 48, bottom].
Maremycin A, a diketopiperazine alkaloid, was isolated from the culture broth of marine Streptomyces species B 9173[122]. The application of a new synthetic strategy for the total synthesis of complex natural products is generally considered an important validation of its synthetic potential and usefulness.
In 2012, Bergonzini et al.[123] established a chiral secondary amine C8-catalyzed enantioselective [3 + 2] annulation of α, β-unsaturated aldehydes 98 with 3-hydroxy oxindoles 69, generating spiro oxindole-butyrolactones 99 and 100 in high enantioselectivities and low diastereoselectivities [Scheme 49A]. Although the diastereoselectivity is not as good as expected, the diastereomerically pure compounds 99 and 100 can be easily separated by silica column chromatography. It is important to note that the described method could be used for the synthesis of the complex natural product Maremycin A in three steps from chiral spirolactone synthon 100 [Scheme 49B]. Thus, this elegant protocol helps elucidate the absolute structure of the chiral spirolactone frameworks, and it is a critical building block in the total synthesis of natural products.
Scheme 49. Stereocontrolled synthesis of spiro oxindole-butyrolactones and maremycin A. This figure is used with permission from John Wiley and Sons Ltd[123].
Although direct activation of the β-Csp3 of saturated carbonyl compounds using NHCs has been successfully developed, due to their chemical inertness, great challenges remain in this field. Saturated acid chlorides are known to be highly reactive and prone to hydrolyze. Thus, activation of the saturated acid chlorides under mild conditions is desired.
In 2019, Zhu et al.[124] developed a new chiral NHC C27 catalyzed annulation of 69 with acyl chloride 104 in the mixture of toluene and DCM (1:1, v/v) at ambient temperature for the synthesis of cyclic spiro product 105 with high yields, excellent diastereo- and enantioselectivities [Scheme 50].
Scheme 50. Spirolactonization reaction of 3-hydroxyoxindoles 69 with 3-substituted saturated acid chlorides 104. This figure is used with permission from the Royal Society of Chemistry[124].
The plausible mechanism for the formation of 105 was proposed. First of all, the β-Csp3 of 3-substituted saturated acid chlorides 104 was activated by the deprotonation to give the intermediate bearing an active electrophilic carbon. Oxidation of Breslow intermediates to the α, β-unsaturated acyl salt intermediate is a key step to the formation of functionalized spirocyclic oxindole-lactones 105.
The spirocyclic oxindole-lactone 105 could be applied to the synthesis of the 3-hydroxy-indole natural product maremycin B according to the literature methods [Scheme 51][125].
Scheme 51. Application of compound 105 in the total synthesis of maremycin B. This figure is used with permission from the Royal Society of Chemistry[124].
Spirolactonization reaction of isatins
The development of efficient strategies for the stereoselective construction of highly modified heterocyclic molecules is always a key goal in chemical synthesis. Isatins, also known as tribulin, are an important class of heterocyclic compounds. The germinal carbonyls are as exceptional electrophiles in asymmetric catalysis. Thus, reaction of isatins with electron-rich species via nucleophilic aldol addition followed by an intramolecular lactonization could give the spirocyclics products [Scheme 52].
Recently, the 3-alkylidene oxindoles could act as vinylogous nucleophiles to functionalize at the γ-position with electrophiles[126,127]. The research involving vinylogous reaction of enolates derived from 3-alkylidene oxindole derivatives is one of the most efficient protocols in organic synthesis. Many efficient and elegant methodologies have been created using 3-alkylidene oxindoles as nucleophiles for the direct formation of allylic compounds, which is a key structural element in a number of natural products and synthetic bioactive molecules.
Pentenelactone scaffolds are widely present in a number of natural and unnatural compounds which possess potent biological activities. Oxindoles are also regarded as one of the privileged skeletons in bioactive compounds; thus, there is an urgent need for the combination of an oxindole scaffold and a pentenelactone motif in one molecule, which will be interesting not only to synthetic but also to medicinal chemists.
In 2016, a direct enantioselective organocatalytic reaction of 3-alkylidene oxindoles 107 with isatins 106 in the presence of C28 was developed by Han et al. for the synthesis of spirocyclic product 108 via vinylogous aldol/ring opening and subsequent intramolecular lactonization [Scheme 53][128].
Scheme 53. Spirolactonization reaction of isatins 106 with 3-alkylidene oxindoles 107. This figure is used with permission from the Royal Society of Chemistry[128].
With the help of alkaloid-squaramide bifunctional organocatalyst C28, the cyclization was initiated with the adol reaction between activated intermediate s-cis enolate A and 106 led to the formation of intermediate B. Subsequent intramolecular lactonization, leading to the cleavage of the oxindole ring and generation of a broad range of enantioenriched spirocyclic oxindole-pentenelactone derivatives 108 in good to excellent yields with high enantioselectivities [Scheme 53].
In recent years, due to the presence of multiple functional groups, the α, β-unsaturated butenolides have been known as versatile candidates for the synthesis of various oxygen-containing heterocyclic analogues[129,130]. The privileged moiety has been found in several bioactive compounds as well.
Similar to the work of Han et al.[128], another synthetic route toward the enantioenriched functionalized spirocyclic oxindole-pentenelactone scaffolds 110 was reported by Wang et al. [Scheme 54][131]. Treatment of α, β-unsaturated butenolides 109 with isatins 106 in the presence of Takemoto’s amine-thiourea catalyst C29 in toluene at 0 °C gave the desired spirocyclic product 110 in good to excellent yields (73%-99%) and enantioselectivities (71%-97% ee).
Scheme 54. Spirolactonization reaction of isatins 106 with α, β-unsaturated butenolides 109. This figure is used with permission from the Royal Society of Chemistry[131].
A similar mechanism of the sequential vinylogous aldol reaction/ring opening and subsequent intramolecular lactonization was proposed. It is important to mention that the intermolecular hydrogen bonding between the C29 and two substates plays a critical role in the control of the stereoselectivity [Scheme 54].
Moreover, in order to further expand the application of α, β-unsaturated butenolides 109 in the construction of chiral spirocyclic pentenelactone scaffolds, the spirolactonization of α, β-unsaturated butenolides 109 with other electrophiles were also attempted. Upon using phenanthrenequinone 111 instead of isatin 106 as an electrophile, desired products 112 and 113 were obtained in excellent yields and good selectivities. So it is an attractive combination of pharmacologically significant phenanthrenequinone and pentenelactone units [Scheme 55].
Scheme 55. Experimental results using phenanthrenequinone 111 as an electrophile. This figure is used with permission from the Royal Society of Chemistry[131].
The hetero-Diels-Alder (HDA) reactions of the carbonyl group with α, β-unsaturated carbonyl has been developed for the rapid generation of six-membered oxygen-containing heterocycles. Azlactones, as versatile reactants, are great candidates for the synthesis of potentially bioactive α,α-disubstituted α-amino acids.
In 2017, Gao et al.[132] reported a catalytic asymmetric hetero-Diels-Alder (HDA) reaction olefinic azlactones 113 with isatins 106 in the presence of C30 in DCE at 30 °C for 48 h for the synthesis of spirocyclic product 114 [Scheme 56].
Scheme 56. Spirolactonization reaction of isatins 106 with olefinic azlactones 113. This figure is used with permission from the Royal Society of Chemistry[132].
It was found that the vinylogous reactivity of azlactones 113 was realized hydrogen-bond directed activation of both of the reaction partners via γ-addition of 113 to 106, and the desired medicinally important spirocyclic oxindole-pentenelactone scaffolds 114 were obtained in good yields and enantioselectivity [Scheme 56].
In recent years, the NHC-bonded intermediates’ generation and their corresponding reaction with polar double bonds moiety of the substrates have drawn a lot of attention since a variety of highly functionalized molecules could be easily obtained in such a simple reaction.
In 2015, using the HOBt esters of carboxylic acids and isatins as substrates, an attractive alternative NHC-catalyzed cascade synthetic route toward the potential biological enantioenriched spirocyclic oxindole-pentenelactone scaffolds was reported by Que et al. [Scheme 57, top][133]. The spirocyclic oxindole-pentenelactones 116 were obtained in good yields with high enantioselectivity.
Scheme 57. Spirolactonization reaction of isatins 106 and HOBt esters of carboxylic acids 115. This figure is used with permission from the American Chemical Society[133].
It was found that the HOBt esters of carboxylic acids can be transformed into the NHC-bonded intermediate, followed by the reaction with activated C-O double bond of isatins 106 provided a new alternative method for the synthesis of spirocyclic oxindole-pentenelactones 116 [Scheme 57, bottom].
Aromatic moiety exists almost in every single bioactive molecule and natural product. The catalytic functionalization of aromatic Csp2 and the attached Csp3 is fundamental to organic chemistry and therefore an important subject of research.
Notably, the compounds containing isatin units, which exhibit interesting biological properties, such as anticonvulsant, antimicrobial, antitumor, antiviral, anti-HIV and antitubercular activities. In 2013, Chen et al.[134] developed an NHC-derivative C25 catalyzed annulation of isatins 106 with indole 3-carboxaldehydes 117 in the presence of quinone oxidant 96 to give spirocyclic lactones 118 containing isatin units in good to excellent yields and ees [Scheme 58].
Scheme 58. Spirolactonization reaction of isatins 106 and α-branched indole 3-carboxaldehydes 117. This figure is used with permission from John Wiley and Sons Ltd[134].
In the proposed reaction mechanism, the C25-catalyzed 96-oxidized activation of the Csp3 of α-methylindole-3-carboxaldehydes 117 to give ortho-quinodimethane intermediates, which undergoes formal [4 + 2] cycloaddition with an isatin 106 to give a spirocyclic oxindole-pentenelactone 118 [Scheme 58]. Both the pre-catalyst C25 and oxidizer 96 play a critical role in the activation of the 117.
Recently, pyrazoleamides have been used as special donors in enantioselective Mannich and Michael reactions. In these reactions, the versatile pyrazoleamide functions as an activating group, a directing group for enhancing stereocontrol, as well as a good leaving group for subsequent transformations.
In 2014, the synthesis of optically active spirocyclic oxindole-pentenelactones 120 was reported by Li et al.[135] via the enantioselective vinylogous aldol-cyclization cascade reaction of allyl pyrazoleamides 119 with isatins 106 in the presence of Takemoto catalyst C29 in acetonitrile at ambient temperature. Various spirocyclic products 120 were obtained in excellent yields (93%-99%) and enantioselectivities (82%-97% ee). It is worth mentioning that the protection of the nitrogen atom in the indole ring is not necessary, although the yield and enantioselectivity of the product 120d are relatively lower. Thus, the reported method establishes a new protocol for the asymmetric construction of structurally novel spirocyclic oxindole-pentenelactones 120 employing β, γ-unsaturated aldehyde derivatives [Scheme 59].
Scheme 59. Spirolactonization reaction of isatins 106 and α-branched indole 3-carboxaldehydes 119. This figure is used with permission from the Royal Society of Chemistry[135].
Recently, Zhang et al.[136] reported a series of NHCs derived from L-pyroglutamic acid. Due to the high enantioselectivity of L-pyroglutamic acid NHCs, in 2011, further exploration of the chiral NHCs catalyzed enantioselective umpolung reactions of enals 121 was performed.
It was found that the proximal hydroxyl group of L-pyroglutamic acid C31 was an efficient catalyst for the activation of isatins 106 by the formation of intermolecular hydrogen bonds between the two geminal carbonyl groups. It also plays a role in directing the addition of the homoenolate to the carbonyl group of isatin 106. Thus, followed by the [3 + 2] annulation of enals 121 and activated isatins 106, the corresponding spirocyclic oxindole-butyrolactones 122 were obtained in good yield with good diastereo- and enantioselectivities [Scheme 60][137].
Scheme 60. Spirolactonization reaction of enals 121 and isatins 106. This figure is used with permission from Wiley-VCH Verlag[137].
In 2011, a chiral N-heterocyclic carbenes C32-catalyzed cyclization of α, β-unsaturated β-methylacyl chlorides 123 with isatins 106 for the synthesis of optically active spirocyclic oxindole-pentenelactones 124 was also developed by Shen et al. [Scheme 61, top][138].
Scheme 61. Spirolactonization reaction of isatins 106 and unsaturated acyl chlorides 123. This figure is used with permission from Wiley-VCH Verlag[138].
The proposed catalytic cycle was described in Scheme 61, bottom part. The addition of in situ generated NHC intermediate to the carbonyl carbon of the vinyl ketene 123', which is generated via base-promoted halo dehydrogenation of the acyl chloride 123, gave the diene intermediate A. The reaction of A and isatin 106 proceeded via either hetero-Diels-Alder reaction or stepwise vinylogous aldol reaction to give the zwitterionic B after a spontaneous intramolecular cyclization B, which could be explained by the formation of the stable six-member ring to reduce the steric hindrance. Regeneration of the NHC catalyst C32 for next catalyst circle led to the formation of spirocyclic oxindole-pentenelactone 124 [Scheme 61, bottom].
Over the last decade, despite significant advancements in the area of N-heterocyclic carbene-catalyzed homoenolate equivalents have achieved, activation of NHC catalysis in the presence of Lewis acid and transition metal catalysis is still underdeveloped for the access of hetero- and carbocyclic structures.
In 2012, the enantioselective annulation of enals 125 with isatins 106 in the presence of cooperative catalysis NHC/Lewis acid (LiCl) in THF at ambient temperature was reported by Sun et al.[137] for the synthesis of spirocyclic oxindole-lactone products 126 [Scheme 62].
Scheme 62. Spirolactonization reaction of isatins 106 and α, β-unsaturated aldehydes 125. This figure is used with permission from the Royal Society of Chemistry[137].
It was proposed that the initial activation of 125 with NHC catalyst C33, while the isatins 106 was activated by LiCl at the same time. Those two activated substrates were further stabilized by the π-π stacking and O-Li-O covalent bonds. The alkali salt significantly enhances the enantioselectivity by securing the confirmation of substrates to give the spirocyclic oxindole-lactone products 126 [Scheme 62, bottom].
More importantly, the total synthesis of Maremycin B was successfully achieved in a few steps via the described highly selective formal [3 + 2] annulation methodology, which is a great example of the possible application for chemists [Scheme 63].
Scheme 63. Total synthesis of maremycin B. This figure is used with permission from the Royal Society of Chemistry[137].
Due to the electronegativity of the fluorine atom, the sigma bond between carbon and fluoride is generally considered the strongest single bond for the carbon atom. Thus, the reduction of fluorocarbons or the transformation of fluorocarbons into non-fluorinated organic molecules under mild conditions is extremely challenging but still has drawn much attention.
In 2018, the reaction of γ-fluoroenals 130 with isatins 106 in the presence of chiral NHC catalyst C34 in toluene at 0 °C gave spirocyclic oxindole-pentenelactone scaffolds 131 in up to 91% yield with up to > 99% ee [Scheme 64][139].
Scheme 64. Spirolactonization reaction of isatins 106 and γ-fluoroenals 130. This figure is used with permission from the Royal Society of Chemistry[139].
Upon the activation of the γ-fluoroenals 130 with in situ generated NHC, the Zwitterion intermediate I was obtained. Followed by the 1,2-H shift and tautomerization, along with the cleavage of the C-F bond, intermediate III was obtained. Sequential 1,5-H shift, base-promoted hydrogen abstraction and [4 + 2] cyclization gave the final spiro product 131. It is worth mentioning that the C-F bond cleaved without the help transition-metal catalyst and elevated temperature was not required. To further understand the mechanism, Density functional theory (DFT) calculations were performed to elucidate the C-F bond cleavage process [Scheme 64].
In 2015, an NHC C25-catalyzed annulation of 106 and aldehyde 132 in THF was developed by Cheng et al.[140]. The corresponding spirocyclic oxindole-pentenelactones 133 were obtained in moderate to good yields and enantioselectivities [Scheme 65].
Scheme 65. Spirolactonization reaction of isatins 106 and γ-preoxidized enal 132. This figure is used with permission from Wiley-VCH Verlag[140].
It is proposed that the addition of in situ generated NHC to 132 initially gives the vinyl Breslow intermediate A, which decomposes to the NHC-bound unsubstituted dienolate B in the presence of base. Subsequently, the γ-addition of the dienolate intermediate B to the isatin 106 affords the acyl azolium adduct C. Finally, the lactonization leads to the desired spirocyclic oxindolodihydropyranone 133 and regenerates the NHC catalyst C25 [Scheme 65].
CONCLUSION AND OUTLOOK
The catalytic asymmetric synthesis of chiral spirolactone skeletons is an important topic in synthetic chemistry and medicinal chemistry. This review has summarized the efficient organocatalytic asymmetric cascade reactions for the synthesis of desired chiral spirolactone molecules with structural diversity and complexity. However, this research is still in its early stage. The below-listed challenges need to be addressed as well. (a) The organocatalytic asymmetric cascade synthesis of chiral spirolactone scaffolds is confined to the two major organocatalytic asymmetric synthetic routes as discussed above. Thus, the constructed scaffolds are mainly limited to spirobutyrolactones and spirovalerolactones derivatives. At the time of this writing, there are no other cascade reactions available for the catalytic asymmetric construction of chiral spiropropyllactones. So, the careful design of specific reagents to develop an efficient and versatile platform for organocatalytic asymmetric cascade synthesis is highly desired. (b) Although spirolactone moiety exists in lots of natural products, the successful application of organocatalytic asymmetric cascade reactions in the total synthesis of natural products is still limited. (c) Although controlling the enantioselectivity in catalytic asymmetric radical reactions remains a great challenge, it is highly valuable to have new activation models for the synthesis of spirolactones by the combination of asymmetric organocatalysis with visible light photoredox and electrochemical synthesis. (d) The biological properties of spirolactone derivatives need to be further explored and there is still extensive room to address the biological activity issues. We are proud to work in this fascinating area and are sure it will be even better with the continuous endeavors of every chemist.
DECLARATIONS
Authors’ contributionsPrepared and corrected the manuscript: Yang J, Pan BW, Chen L, Zhou Y, Liu XL
Availability of data and materialsNot applicable.
Financial support and sponsorshipThe research was supported by the NSFC (22061006) and Project of Guizhou province (ZK[2022]480, ZK[2022]144, KY[2022]253).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Quasdorf KW, Overman LE. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 2014;516:181-91.
2. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 2002;45:2615-23.
3. Hazra A, Bharitkar YP, Chakraborty D, et al. Regio- and stereoselective synthesis of a library of bioactive dispiro-oxindolo/acenaphthoquino andrographolides
4. Hu R, Huang JL, Yuan FY, et al. Crotonianoids A-C, Three unusual tigliane diterpenoids from the seeds of croton tiglium and their anti-prostate cancer activity. J Org Chem 2022;87:9301-6.
5. Liu J, Flegel J, Otte F, et al. Combination of pseudo-natural product design and formal natural product ring distortion yields stereochemically and biologically diverse pseudo-sesquiterpenoid alkaloids. Angew Chem Int Ed Engl 2021;60:21384-95.
6. Alemán J, Cabrera S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem Soc Rev 2013;42:774-93.
7. Zong L, Tan CH. Phase-transfer and ion-pairing catalysis of pentanidiums and bisguanidiniums. Acc Chem Res 2017;50:842-56.
8. Metrano AJ, Miller SJ. Peptide-based catalysts reach the outer sphere through remote desymmetrization and atroposelectivity. Acc Chem Res 2019;52:199-215.
9. Zhang YC, Jiang F, Shi F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach. Acc Chem Res 2020;53:425-46.
10. Ramachary DB, Jain S. Sequential one-pot combination of multi-component and multi-catalysis cascade reactions: an emerging technology in organic synthesis. Org Biomol Chem 2011;9:1277-300.
13. Dondoni A, Massi A. Asymmetric organocatalysis: from infancy to adolescence. Angew Chem Int Ed Engl 2008;47:4638-60.
14. Zhang HH, Shi F. Organocatalytic atroposelective synthesis of indole derivatives bearing axial chirality: strategies and applications. Acc Chem Res 2022;55:2562-80.
15. Volla CM, Atodiresei I, Rueping M. Catalytic C-C bond-forming multi-component cascade or domino reactions: pushing the boundaries of complexity in asymmetric organocatalysis. Chem Rev 2014;114:2390-431.
16. Nielsen TE, Schreiber SL. Towards the optimal screening collection: a synthesis strategy. Angew Chem Int Ed Engl 2008;47:48-56.
17. Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science 2009;325:161-5.
18. Kumar K, Waldmann H. Synthesis of natural product inspired compound collections. Angew Chem Int Ed Engl 2009;48:3224-42.
19. Koch MA, Schuffenhauer A, Scheck M, et al. Charting biologically relevant chemical space: a structural classification of natural products (SCONP). Proc Natl Acad Sci USA 2005;102:17272-7.
21. Piacente S, Montoro P, Oleszek W, Pizza C. Yucca schidigera bark: phenolic constituents and antioxidant activity. J Nat Prod 2004;67:882-5.
22. Wada S, Hitomi T, Tanaka R. Phenolic compounds Isolated from the bark of Abies sachalinensis. HCA 2009;92:1610-20.
23. Wada S, Hitomi T, Tokuda H, Tanaka R. Anti-tumor-initiating effects of spiro-biflavonoids from Abies sachalinensis. Chem Biodivers 2010;7:2303-8.
24. Vedejs E, Daugulis O, Diver ST. Enantioselective acylations catalyzed by chiral phosphines. J Org Chem 1996;61:430-1.
25. Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. Asymmetric [3 + 2] cycloaddition of 2,3-butadienoates with electron-deficient olefins catalyzed by novel chiral 2,5-dialkyl-7-phenyl-7- phosphabicyclo[2.2.1]heptanes. J Am Chem Soc 1997;119:3836-7.
26. Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. Asymmetric formation of quaternary carbon centers catalyzed by novel chiral 2,5-dialkyl-7-phenyl-7-phosphabicyclo[2.2.1]heptanes. J Org Chem 1998;63:5631-5.
27. Vedejs E, Daugulis O. 2-aryl-4,4,8-trimethyl-2-phosphabicyclo[3.3.0]octanes: reactive chiral phosphine catalysts for enantioselective acylation. J Am Chem Soc 1999;121:5813-4.
28. Shaw SA, Aleman P, Vedejs E. Development of chiral nucleophilic pyridine catalysts: applications in asymmetric quaternary carbon synthesis. J Am Chem Soc 2003;125:13368-9.
29. Shi M, Chen LH, Li CQ. Chiral phosphine lewis bases catalyzed asymmetric aza-Baylis-Hillman reaction of N-sulfonated imines with activated olefins. J Am Chem Soc 2005;127:3790-800.
30. Wurz RP, Fu GC. Catalytic asymmetric synthesis of piperidine derivatives through the [4 + 2] annulation of imines with allenes. J Am Chem Soc 2005;127:12234-5.
32. Albertshofer K, Tan B, Barbas CF 3rd. Asymmetric construction of spirocyclopentenebenzofuranone core structures
33. Wang D, Wang GP, Sun YL, et al. Chiral phosphine-catalyzed tunable cycloaddition reactions of allenoates with benzofuranone-derived olefins for a highly regio-, diastereo- and enantioselective synthesis of spiro-benzofuranones. Chem Sci 2015;6:7319-25.
34. Ren L, Lei T, Ye J, Gong L. Corrigendum: step-economical synthesis of tetrahydroquinolines by asymmetric relay catalytic friedlander condensation/transfer hydrogenation. Angew Chem Int Ed 2014;53:6027-6027.
35. Sahani RL, Liu RS. Gold-catalyzed [4 + 2] annulation/cyclization cascades of benzisoxazoles with propiolate derivatives to access highly oxygenated tetrahydroquinolines. Angew Chem Int Ed Engl 2017;56:12736-40.
36. Xie M, Chen X, Zhu Y, et al. Asymmetric three-component inverse electron-demand aza-diels-alder reaction: efficient synthesis of ring-fused tetrahydroquinolines. Angew Chem Int Ed Engl 2010;49:3799-802.
37. Zhang JL, Ma R, Zhao HH, Xu PF. Enantioselective construction of spiro-tetrahydroquinoline scaffolds through asymmetric catalytic cascade reactions. Chem Commun (Camb) 2022;58:3493-6.
38. Masters KS, Bräse S. Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis. Chem Rev 2012;112:3717-76.
39. Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Prod Rep 2011;28:1208-28.
40. Shim SH, Baltrusaitis J, Gloer JB, Wicklow DT. Phomalevones A-C: dimeric and pseudodimeric polyketides from a fungicolous Hawaiian isolate of Phoma sp. (Cucurbitariaceae). J Nat Prod 2011;74:395-401.
41. Goel R, Sharma V, Budhiraja A, Ishar MP. Synthesis and evaluation of novel 3a,9a-dihydro-1-ethoxycarbonyl-1-cyclopenteno[5,4-b]benzopyran-4-ones as antifungal agents. Bioorg Med Chem Lett 2012;22:4665-7.
42. Zhang F, Li L, Niu S, et al. A thiopyranchromenone and other chromone derivatives from an Endolichenic fungus, Preussia africana. J Nat Prod 2012;75:230-7.
43. Zhang M, Gong Y, Zhou W, Zhou Y, Liu X. Recent advances of chromone-based reactants in the catalytic asymmetric domino annulation reaction. Org Chem Front 2021;8:3968-89.
44. Liu X, Zuo X, Wang J, Chang S, Wei Q, Zhou Y. A bifunctional pyrazolone–chromone synthon directed organocatalytic double Michael cascade reaction: forging five stereocenters in structurally diverse hexahydroxanthones. Org Chem Front 2019;6:1485-90.
45. Liu XL, Zhou G, Gong Y, et al. Stereocontrolled synthesis of bispirooxindole-based hexahydroxanthones with five contiguous stereocenters. Org Lett 2019;21:2528-31.
46. Guo DG, Wang HJ, Zhou Y, Liu XL. Advances in chromone-based reactants in the ring opening and skeletal reconstruction reaction: access to skeletally diverse salicyloylbenzene/heterocycle derivatives. Org Biomol Chem 2022;20:4681-98.
47. Chang SQ, Zou X, Gong Y, He XW, Liu XL, Zhou Y. Stereocontrolled construction of six vicinal stereogenic centers on a hexahydroxanthone framework through a formal [2+1+3] annulation. Chem Commun (Camb) 2019;55:14003-6.
48. Li X, Lin MH, Han Y, et al. Asymmetric Diels-Alder reaction of 3-olefinic benzofuran-2-ones and polyenals: construction of chiral spirocyclic benzofuran-2-ones. Org Lett 2014;16:114-7.
49. Zhou Q, Xiao Y, Yuan X, Chen Y. Asymmetric diels-alder reactions of 2,4,6-trienals
50. Cassani C, Tian X, Escudero-Adán EC, Melchiorre P. Multiple approaches to enantiopure spirocyclic benzofuranones using organocatalytic cascade reactions. Chem Commun (Camb) 2011;47:233-5.
51. Chatterjee I, Bastida D, Melchiorre P. Vinylogous organocatalytic triple cascade reaction: forging six stereocenters in complex spiro-oxindolic cyclohexanes. Adv Synth Catal 2013;355:3124-30.
52. Chintalapudi V, Galvin EA, Greenaway RL, Anderson EA. Combining cycloisomerization with trienamine catalysis: a regiochemically flexible enantio- and diastereoselective synthesis of hexahydroindoles. Chem Commun (Camb) 2016;52:693-6.
53. Cao Y, Jiang X, Liu L, Shen F, Zhang F, Wang R. Enantioselective Michael/cyclization reaction sequence: scaffold-inspired synthesis of spirooxindoles with multiple stereocenters. Angew Chem Int Ed Engl 2011;50:9124-7.
54. Zhang L, Quan W, Liu R, Tian Y, Pan B, Liu X. Diastereoselective construction of a library of structural bispiro[butyrolactone/valerolactone-pyrrolidin-indanedione] hybrids
55. Wang ZH, Wu ZJ, Yue DF, et al. Organocatalytic asymmetric [3+2] cycloaddition of
56. Zhou F, Zhu L, Pan BW, Shi Y, Liu YL, Zhou J. Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem Sci 2020;11:9341-65.
57. Steven A, Overman LE. Total synthesis of complex cyclotryptamine alkaloids: stereocontrolled construction of quaternary carbon stereocenters. Angew Chem Int Ed Engl 2007;46:5488-508.
58. Büschleb M, Dorich S, Hanessian S, Tao D, Schenthal KB, Overman LE. Synthetic strategies toward natural products containing contiguous stereogenic quaternary carbon atoms. Angew Chem Int Ed Engl 2016;55:4156-86.
59. Long R, Huang J, Gong J, Yang Z. Direct construction of vicinal all-carbon quaternary stereocenters in natural product synthesis. Nat Prod Rep 2015;32:1584-601.
60. Companyó X, Zea A, Alba AN, Mazzanti A, Moyano A, Rios R. Organocatalytic synthesis of spiro compounds
61. Li X, Yang C, Jin JL, Xue XS, Cheng JP. Synthesis of optically enriched spirocyclic benzofuran-2-ones by bifunctional thiourea-base catalyzed double-Michael addition of benzofuran-2-ones to dienones. Chem Asian J 2013;8:997-1003.
62. Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002;40:803-16.
63. Dawson RM. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol 1994;14:317-31.
64. Zhang M, Wang J, Chang S, Liu X, Zuo X, Zhou Y. Highly efficient enantioselective synthesis of bispiro[benzofuran-oxindole/benzofuran-chromanone]s through organocatalytic inter-/intramolecular Michael cycloaddition. Chinese Chem Lett 2020;31:381-5.
65. Itazaki H, Nagashima K, Kawamura Y, Matsumoto K, Nakai H, Terui Y. Cinatrins, a novel family of phospholipase A2 inhibitors. I. Taxonomy and fermentation of the producing culture; isolation and structures of cinatrins. J Antibiot (Tokyo) 1992;45:38-49.
66. Tanaka K, Itazaki H, Yoshida T. Cinatrins, a novel family of phospholipase A2 inhibitors. II. Biological activities. J Antibiot (Tokyo) 1992;45:50-5.
67. Keyzers RA, Daoust J, Davies-Coleman MT, et al. Autophagy-modulating aminosteroids isolated from the sponge Cliona celata. Org Lett 2008;10:2959-62.
68. Machida K, Kikuchi M. Studies on the constituents of viburnum species. VIII. GAMMA.-lactone glycosides from the leaves of viburnum wrightii MIQ. Chem Pharm Bull 1994;42:1388-92.
69. Li XL, Cheng X, Yang LM, et al. Dichotomains A and B: two new highly oxygenated phenolic derivatives from Dicranopteris dichotoma. Org Lett 2006;8:1937-40.
70. Perold GW, Pachler KGR. The structure and chemistry of leucodrin. J Chem Soc , C 1966; doi: 10.1039/j39660001918.
71. Wang ZD, Wang F, Li X, Cheng JP.
72. Jiang X, Cao Y, Wang Y, Liu L, Shen F, Wang R. A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J Am Chem Soc 2010;132:15328-33.
73. Chen WB, Wu ZJ, Hu J, Cun LF, Zhang XM, Yuan WC. Organocatalytic direct asymmetric aldol reactions of 3-isothiocyanato oxindoles to ketones: stereocontrolled synthesis of spirooxindoles bearing highly congested contiguous tetrasubstituted stereocenters. Org Lett 2011;13:2472-5.
74. Lin Y, Liu L, Du D. Squaramide-catalyzed asymmetric Michael/cyclization cascade reaction of 3-isothiocyanato oxindoles with chalcones for synthesis of pyrrolidinyl spirooxindoles. Org Chem Front 2017;4:1229-38.
75. Zhao H, Tian T, Pang H, et al. Organocatalytic [3+2] cycloadditions of barbiturate-based olefins with 3-isothiocyanato oxindoles: highly diastereoselective and enantioselective synthesis of dispirobarbiturates. Adv Synth Catal 2016;358:2619-30.
76. Du D, Xu Q, Li XG, Shi M. Construction of spirocyclic oxindoles through regio- and stereoselective [3+2] or [3+2]/[4+2] cascade reaction of
77. Wang L, Yang D, Li D, et al. Catalytic Asymmetric [3 + 2] cyclization reactions of 3-isothiocyanato oxindoles and alkynyl ketones
78. Kayal S, Mukherjee S. Catalytic Aldol-Cyclization Cascade of 3-Isothiocyanato Oxindoles with
79. Wang L, Yang D, Li D, Wang R. Catalytic enantioselective ring-opening and ring-closing reactions of 3-isothiocyanato oxindoles and N-(2-Picolinoyl)aziridines. Org Lett 2015;17:3004-7.
80. Jiang X, Wang Y, Zhang G, et al. Enantioselective synthesis of cyclic thioureas
81. Liu RM, Zhang M, Han XX, et al. Catalytic asymmetric Michael/cyclization reaction of 3-isothiocyanato thiobutyrolactone: an approach to the construction of a library of bispiro[pyrazolone-thiobutyrolactone] skeletons. Org Biomol Chem 2022;20:5060-5.
82. Kumar V, Kaur K, Gupta GK, Sharma AK. Pyrazole containing natural products: synthetic preview and biological significance. Eur J Med Chem 2013;69:735-53.
83. Chauhan P, Mahajan S, Enders D. Asymmetric synthesis of pyrazoles and pyrazolones employing the reactivity of pyrazolin-5-one derivatives. Chem Commun (Camb) 2015;51:12890-907.
84. Kuo SC, Huang LJ, Nakamura H. Studies on heterocyclic compounds. 6. Synthesis and analgesic and antiinflammatory activities of 3,4-dimethylpyrano[2,3-c]pyrazol-6-one derivatives. J Med Chem 1984;27:539-44.
85. Wilde F, Specker E, Neuenschwander M, Nazaré M, Bodtke A, Link A. Tractable synthesis of multipurpose screening compounds with under-represented molecular features for an open access screening platform. Mol Divers 2014;18:483-95.
86. Kakiuchi Y, Sasaki N, Satoh-Masuoka M, Murofushi H, Murakami-Murofushi K. A novel pyrazolone, 4,4-dichloro-1-(2,4-dichlorophenyl)-3-methyl-5-pyrazolone, as a potent catalytic inhibitor of human telomerase. Biochem Biophys Res Commun 2004;320:1351-8.
87. Liu S, Bao X, Wang B. Pyrazolone: a powerful synthon for asymmetric diverse derivatizations. Chem Commun (Camb) 2018;54:11515-29.
88. Wang L, Shi XM, Dong WP, Zhu LP, Wang R. Efficient construction of highly functionalized spiro[γ-butyrolactone-pyrrolidin-3,3’-oxindole] tricyclic skeletons
89. Chen N, Zhu L, Gan L, et al. Asymmetric synthesis of bispiro[γ-butyrolactone-pyrrolidin-4,4’-pyrazolone] scaffolds containing two quaternary spirocenters
90. Kowalczyk-Dworak D, Albrecht Ł.
91. Guo D, Li Z, Han X, Zhang L, Zhang M, Liu X. Decarboxylative, diastereoselective and exo-selective 1,3-dipolar cycloaddition for diversity-oriented construction of structural spiro[butyrolactone–pyrrolidine–chromanone] hybrids. Synlett 2021;32:1447-52.
92. Guo Y, Meng C, Liu X, et al. Successive waste as reagent: two more steps forward in a pinnick oxidation. Org Lett 2018;20:913-6.
93. Mostinski Y, Lankri D, Tsvelikhovsky D. Transition-metal-catalyzed synthesis of spirolactones. Synthesis 2017;49:2361-73.
94. Hazra A, Paira P, Sahu KB, et al. Chemistry of andrographolide: formation of novel di-spiropyrrolidino and di-spiropyrrolizidino-oxindole adducts
95. Cui BD, Zuo J, Zhao JQ, et al. Tandem Michael addition-ring transformation reactions of 3-hydroxyoxindoles/3-aminooxindoles with olefinic azlactones: direct access to structurally diverse spirocyclic oxindoles. J Org Chem 2014;79:5305-14.
96. Chen L, Wu ZJ, Zhang ML, et al. Organocatalytic asymmetric michael/cyclization cascade reactions of 3-hydroxyoxindoles/3-aminooxindoles with
97. Ma SS, Mei WL, Guo ZK, et al. Two new types of bisindole alkaloid from Trigonostemon lutescens. Org Lett 2013;15:1492-5.
98. Yu B, Yu DQ, Liu HM. Spirooxindoles: promising scaffolds for anticancer agents. Eur J Med Chem 2015;97:673-98.
99. Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev 2008;37:320-30.
100. O’hagan D. Fluorine in health care: Organofluorine containing blockbuster drugs. J Fluorine Chem 2010;131:1071-81.
101. Wang J, Sánchez-Roselló M, Aceña JL, et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem Rev 2014;114:2432-506.
102. Yang ZT, Zhao J, Yang WL, Deng WP. Enantioselective construction of CF3-containing spirooxindole γ-lactones
103. Ming S, Zhao BL, Du DM. Chiral squaramide-catalysed enantioselective Michael/cyclization cascade reaction of 3-hydroxyoxindoles with
104. Zhu SJ, Hao ZF, Pan Y, et al. Asymmetric formal (3 + 2) cyclocondensation of coumarin-3-formylpyrazoles as 3-carbon partners with 3-hydroxyoxindoles
105. Lee KY, Park DY, Kim JN. Synthesis of
106. Kitson RR, Millemaggi A, Taylor RJ. The renaissance of alpha-methylene-gamma-butyrolactones: new synthetic approaches. Angew Chem Int Ed Engl 2009;48:9426-51.
107. Elford T, Hall D. Advances in 2-(alkoxycarbonyl)allylboration of carbonyl compounds and other direct methods for the preparation of
108. Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-κB transcription factor. Cell 1988;53:211-7.
109. Ruben SM, Dillon PJ, Schreck R, et al. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science 1991;254:11.
110. Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J 1991;10:3805-17.
111. Konaklieva MI, Plotkin BJ. Lactones: generic inhibitors of enzymes? Mini Rev Med Chem 2005;5:73-95.
112. Wang QL, Peng L, Wang FY, et al. An organocatalytic asymmetric sequential allylic alkylation-cyclization of Morita-Baylis-Hillman carbonates and 3-hydroxyoxindoles. Chem Commun (Camb) 2013;49:9422-4.
113. Jayakumar S, Muthusamy S, Prakash M, Kesavan V. Enantioselective synthesis of spirooxindole
114. White NA, Rovis T. Enantioselective
115. Zhang Y, Du Y, Huang Z, et al.
116. White NA, Rovis T. Oxidatively initiated NHC-catalyzed enantioselective synthesis of 3,4-disubstituted cyclopentanones from enals. J Am Chem Soc 2015;137:10112-5.
117. Chen XY, Chen KQ, Sun DQ, Ye S.
118. Song ZY, Chen KQ, Chen XY, Ye S. Diastereo- and enantioselective synthesis of spirooxindoles with contiguous tetrasubstituted stereocenters
119. Mahatthananchai J, Bode JW. On the mechanism of
120. Sarkar S, Biswas A, Samanta RC, Studer A. Catalysis with
121. Mukherjee S, Joseph S, Bhunia A, Gonnade RG, Yetra SR, Biju AT. Enantioselective synthesis of spiro γ-butyrolactones by
122. Ueda T, Inada M, Okamoto I, Morita N, Tamura O. Synthesis of maremycins A and D1
123. Bergonzini G, Melchiorre P. Dioxindole in asymmetric catalytic synthesis: routes to enantioenriched 3-substituted 3-hydroxyoxindoles and the preparation of maremycin A. Angew Chem Int Ed Engl 2012;51:971-4.
124. Zhu SY, Zhang H, Ma QW, Liu D, Hui XP. Oxidative NHC catalysis: direct activation of
125. Dugal-tessier J, O'bryan EA, Schroeder TBH, Cohen DT, Scheidt KA. An
126. Curti C, Rassu G, Zambrano V, et al. Bifunctional cinchona alkaloid/thiourea catalyzes direct and enantioselective vinylogous Michael addition of 3-alkylidene oxindoles to nitroolefins. Angew Chem Int Ed Engl 2012;51:6200-4.
127. Rassu G, Zambrano V, Pinna L, et al. Direct regio-, diastereo-, and enantioselective vinylogous michael addition of prochiral 3-alkylideneoxindoles to nitroolefins. Adv Synth Catal 2013;355:1881-6.
128. Han JL, Chang CH. An asymmetric assembly of spirooxindole dihydropyranones through a direct enantioselective organocatalytic vinylogous aldol-cyclization cascade reaction of 3-alkylidene oxindoles with isatins. Chem Commun (Camb) 2016;52:2322-5.
129. Harris JM, O'Doherty GA. Enantioselective syntheses of isoaltholactone, 3-epi-altholactone, and 5-hydroxygoniothalamin. Org Lett 2000;2:2983-6.
130. Yoshimura F, Torizuka M, Mori G, Tanino K. Intramolecular conjugate addition of
131. Wang ZH, Zhang XY, Lei CW, Zhao JQ, You Y, Yuan WC. Highly enantioselective sequential vinylogous aldol reaction/transesterification of methyl-substituted olefinic butyrolactones with isatins for the construction of chiral spirocyclic oxindole-dihydropyranones. Chem Commun (Camb) 2019;55:9327-30.
132. Gao TP, Lin JB, Hu XQ, Xu PF. A catalytic asymmetric hetero-Diels-Alder reaction of olefinic azlactones and isatins: facile access to chiral spirooxindole dihydropyranones. Chem Commun (Camb) 2014;50:8934-6.
133. Que Y, Li T, Yu C, Wang XS, Yao C. Enantioselective assembly of spirocyclic oxindole-dihydropyranones through NHC-catalyzed cascade reaction of isatins with N-hydroxybenzotriazole esters of
134. Chen X, Yang S, Song BA, Chi YR. Corrigendum: functionalization of benzylic C(sp3)-H bonds of heteroaryl aldehydes through
135. Li TZ, Jiang Y, Guan YQ, Sha F, Wu XY. Direct enantioselective vinylogous aldol-cyclization cascade reaction of allyl pyrazoleamides with isatins: asymmetric construction of spirocyclic oxindole-dihydropyranones. Chem Commun (Camb) 2014;50:10790-2.
136. Zhang YR, He L, Wu X, Shao PL, Ye S. Chiral
137. Sun LH, Shen LT, Ye S. Highly diastereo- and enantioselective NHC-catalyzed [3+2] annulation of enals and isatins. Chem Commun (Camb) 2011;47:10136-8.
138. Shen L, Shao P, Ye S.
139. Yan J, Shi K, Zhao C, et al. NHC-catalyzed [4+2] cycloaddition reactions for the synthesis of 3’-spirocyclic oxindoles
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yang J, Pan BW, Chen L, Zhou Y, Liu XL. Recent advances in organocatalytic cascade reactions for enantioselective synthesis of chiral spirolactone skeletons. Chem Synth 2023;3:7. http://dx.doi.org/10.20517/cs.2022.38
AMA Style
Yang J, Pan BW, Chen L, Zhou Y, Liu XL. Recent advances in organocatalytic cascade reactions for enantioselective synthesis of chiral spirolactone skeletons. Chemical Synthesis. 2023; 3(1): 7. http://dx.doi.org/10.20517/cs.2022.38
Chicago/Turabian Style
Yang, Jun, Bo-Wen Pan, Lin Chen, Ying Zhou, Xiong-Li Liu. 2023. "Recent advances in organocatalytic cascade reactions for enantioselective synthesis of chiral spirolactone skeletons" Chemical Synthesis. 3, no.1: 7. http://dx.doi.org/10.20517/cs.2022.38
ACS Style
Yang, J.; Pan B.W.; Chen L.; Zhou Y.; Liu X.L. Recent advances in organocatalytic cascade reactions for enantioselective synthesis of chiral spirolactone skeletons. Chem. Synth. 2023, 3, 7. http://dx.doi.org/10.20517/cs.2022.38
About This Article
Special Issue
Copyright
Author Biographies





Data & Comments
Data
 Cite This Article 49 clicks
Cite This Article 49 clicks


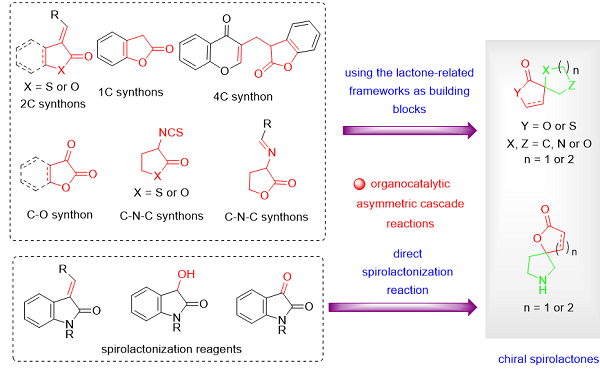
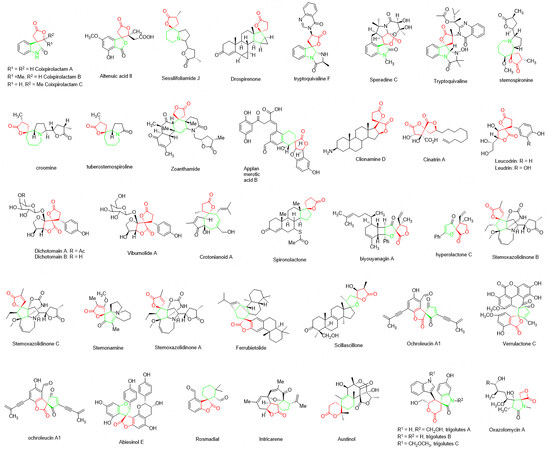
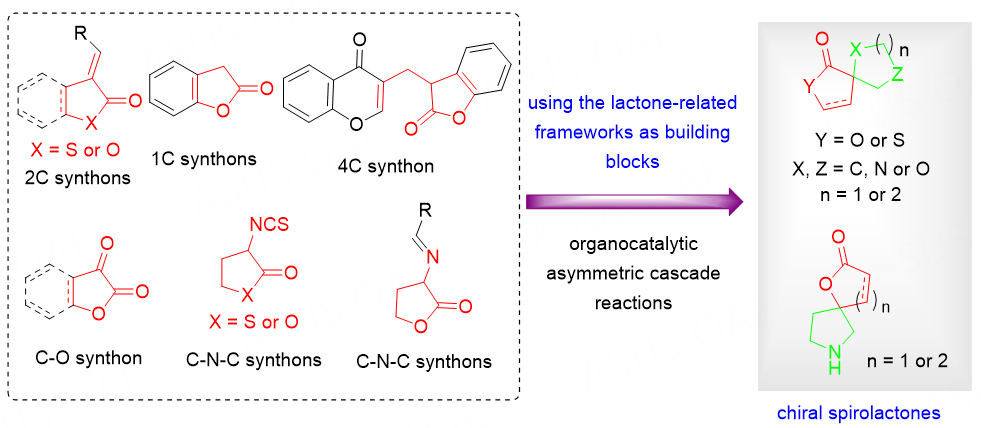
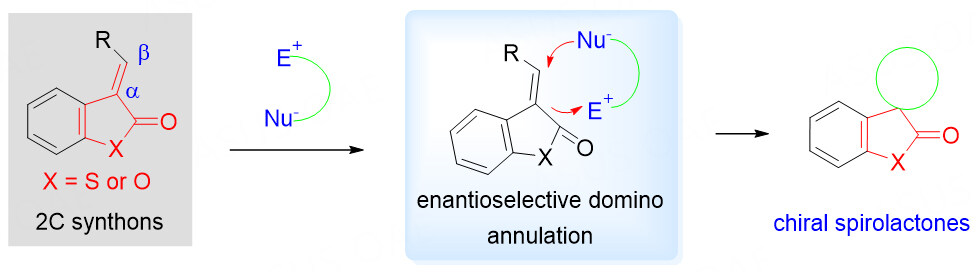
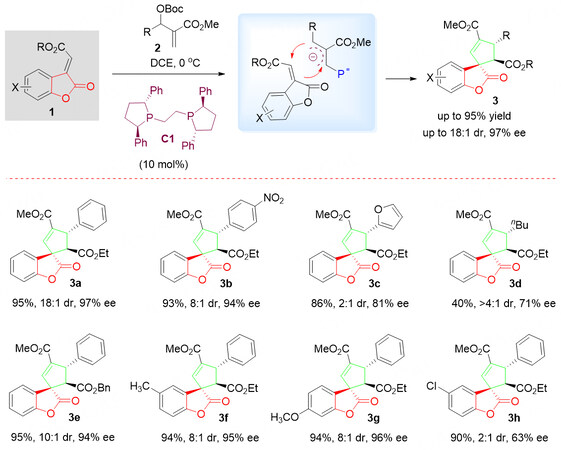
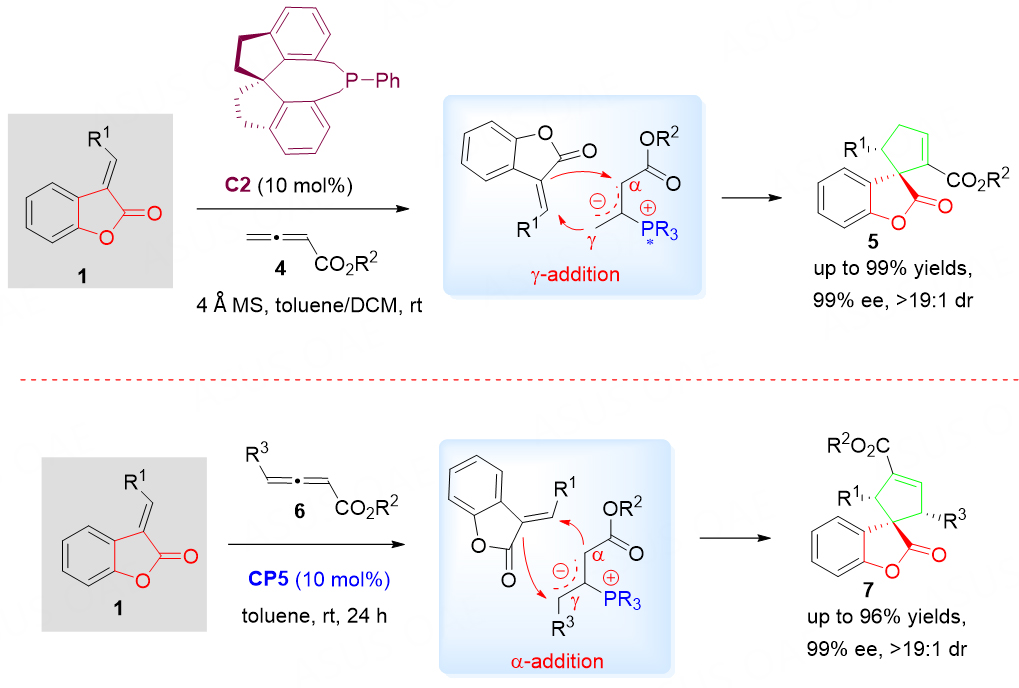
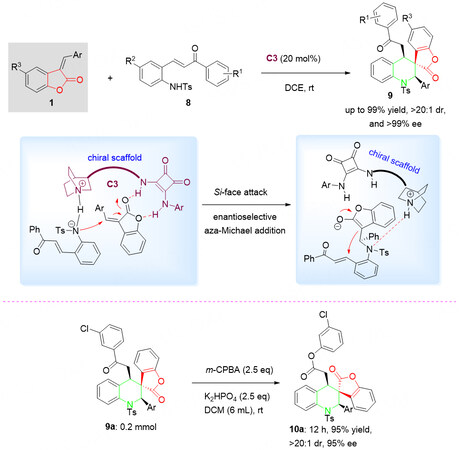

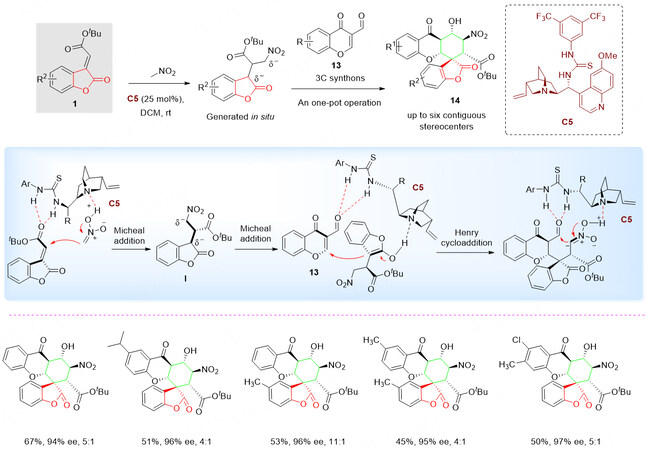
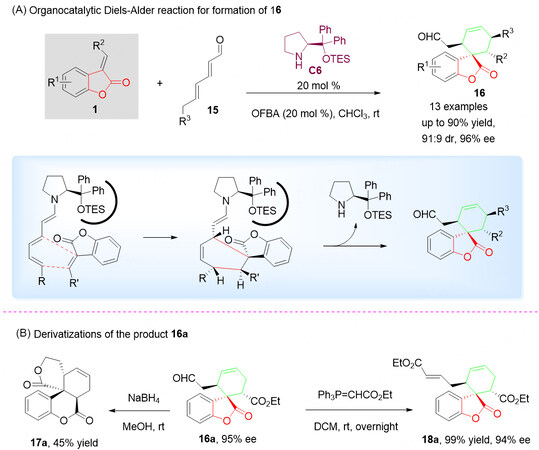
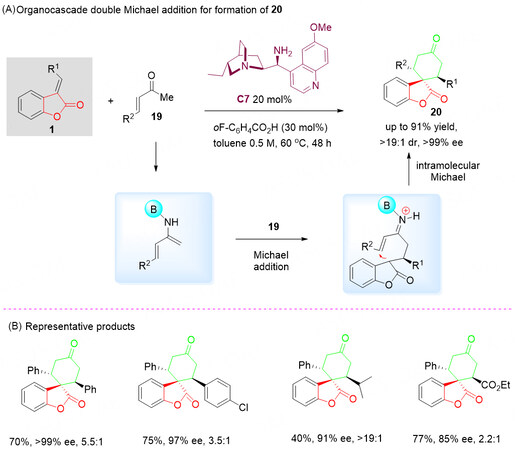
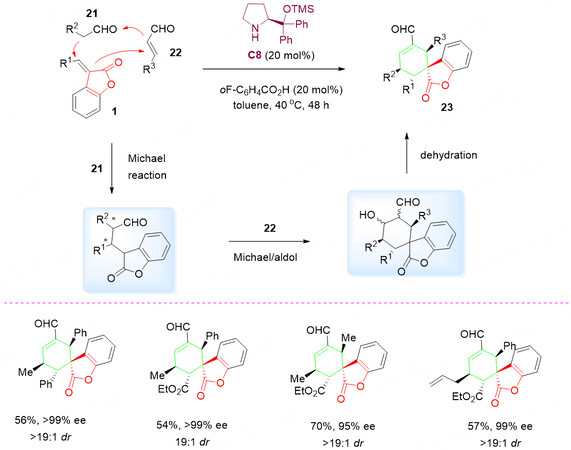
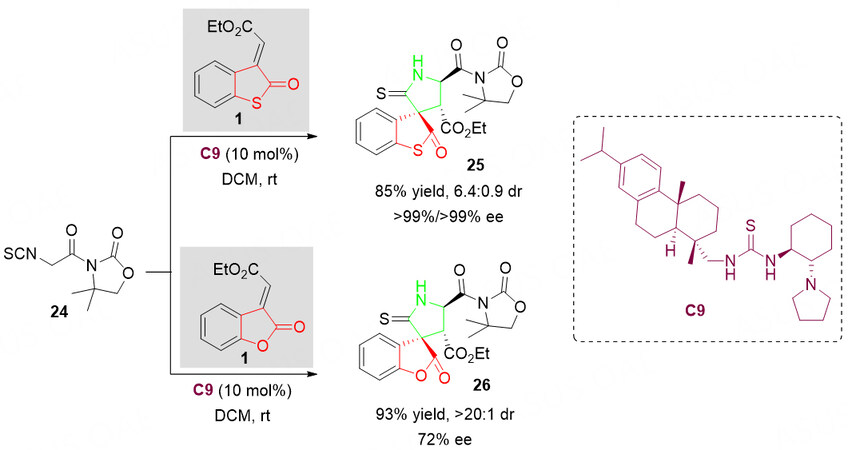
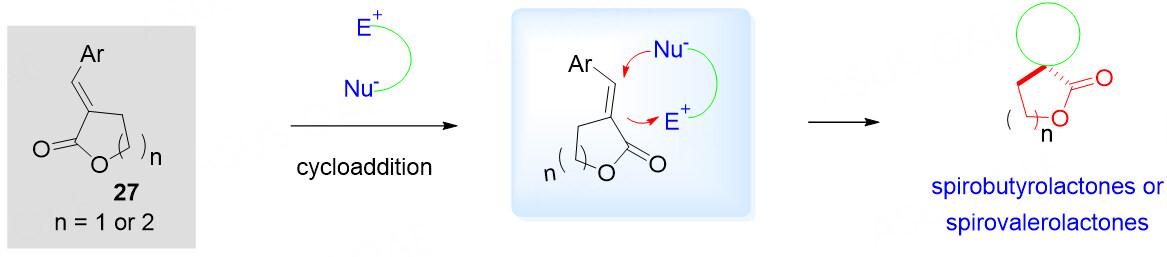
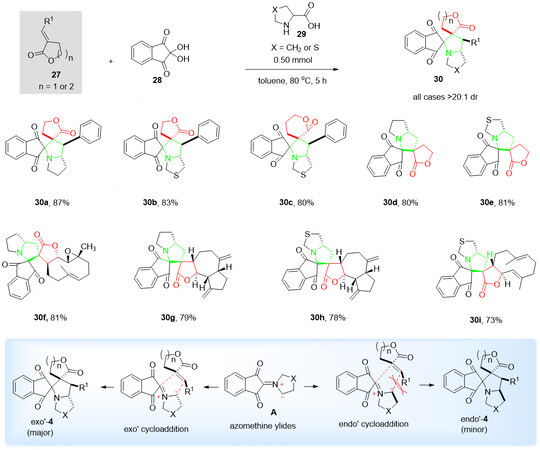

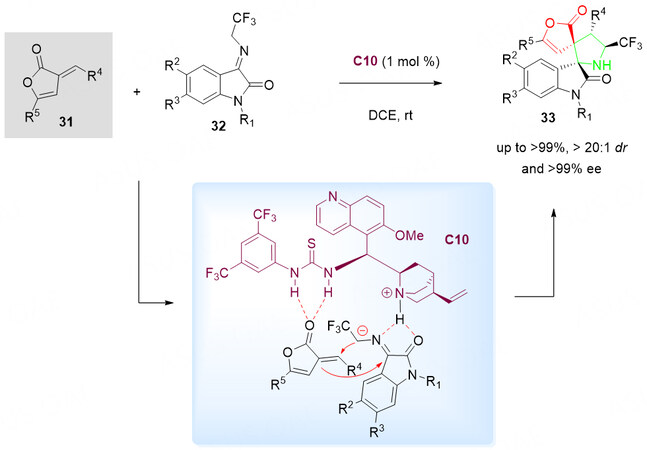
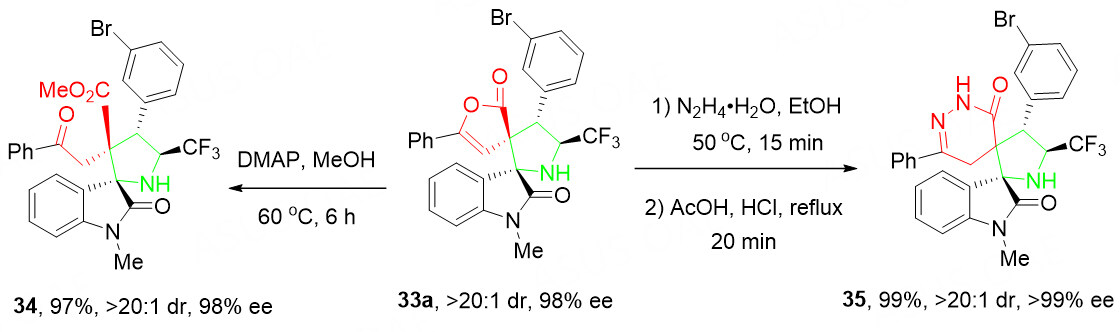
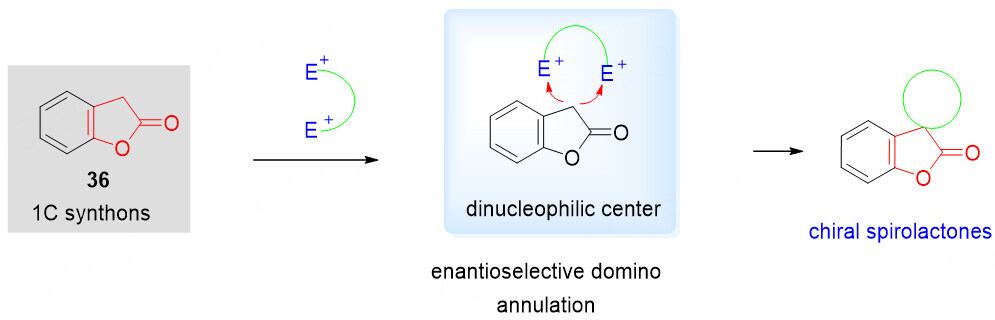
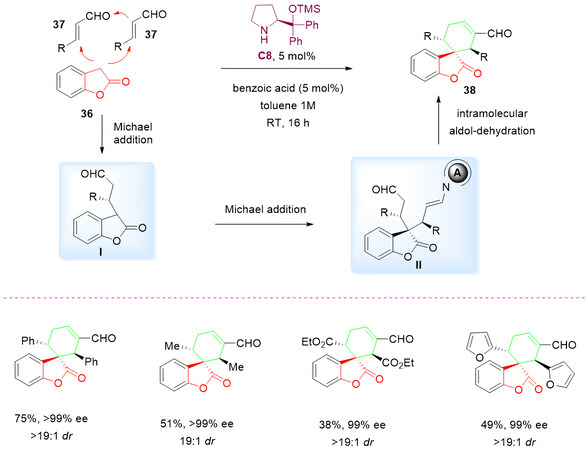
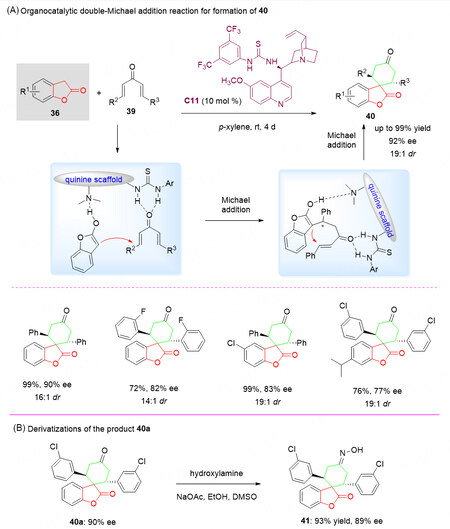
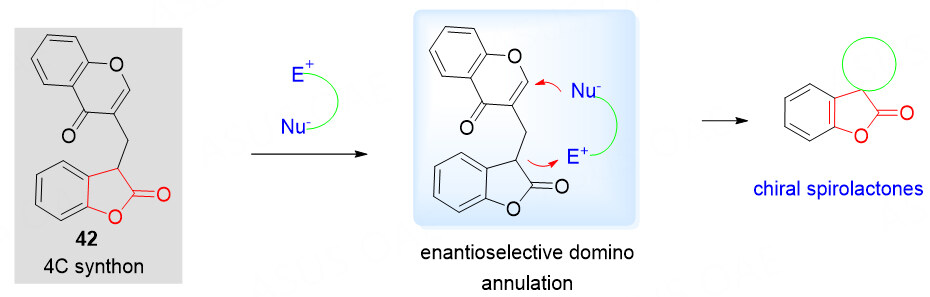
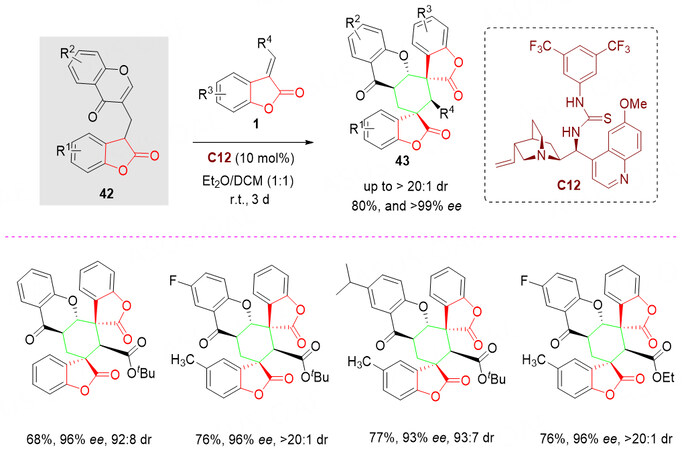
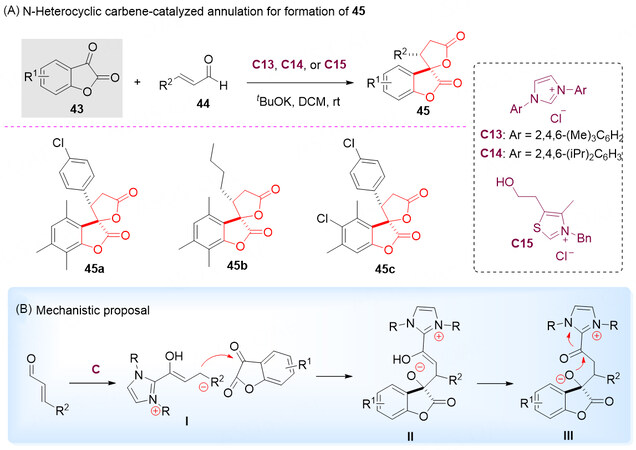
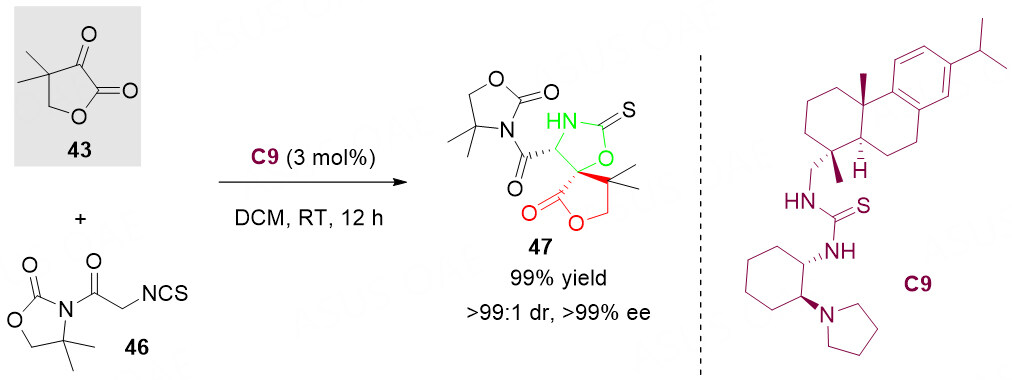
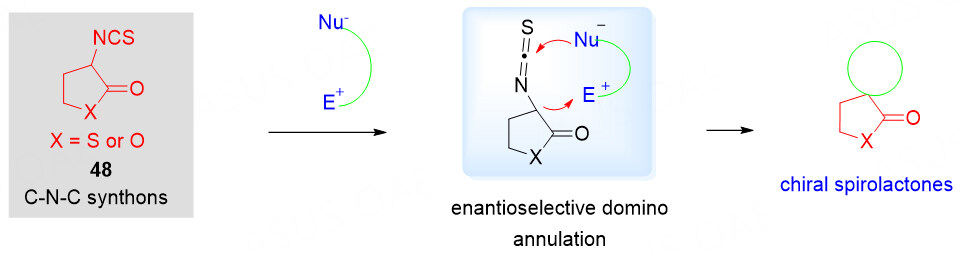
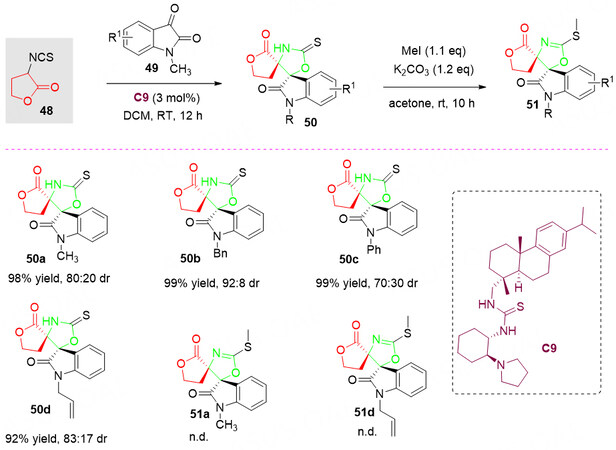
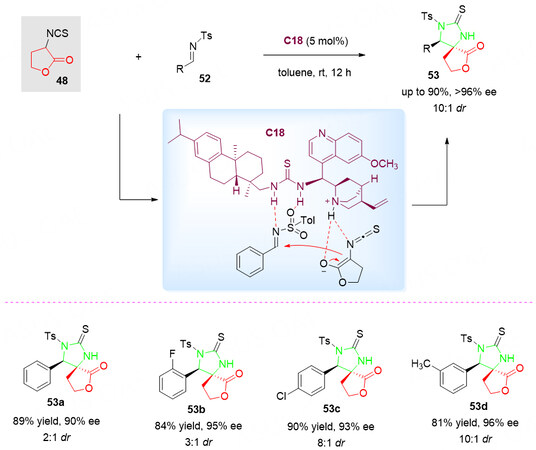
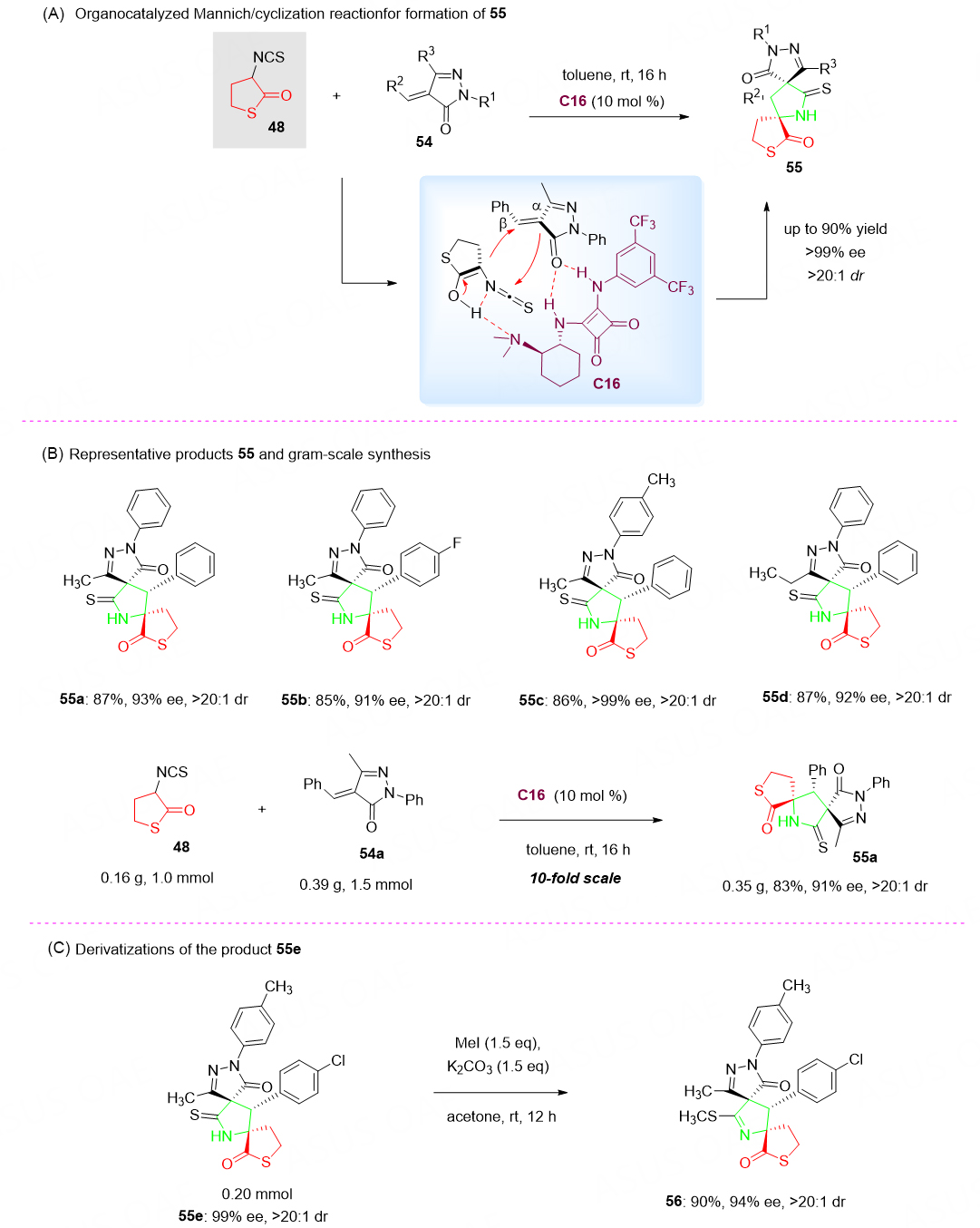
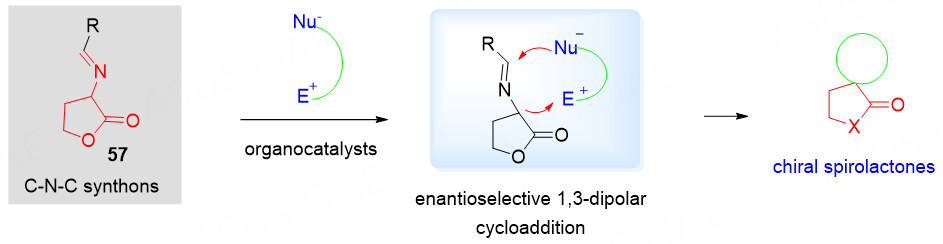
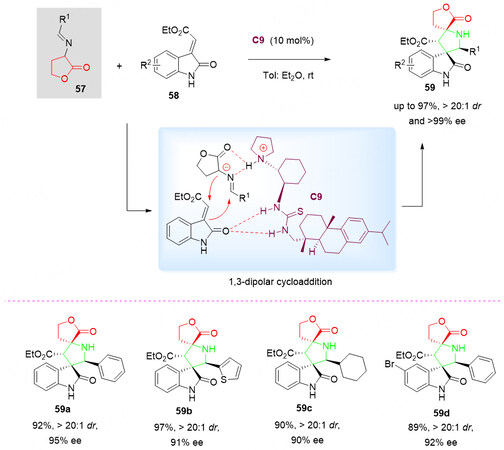
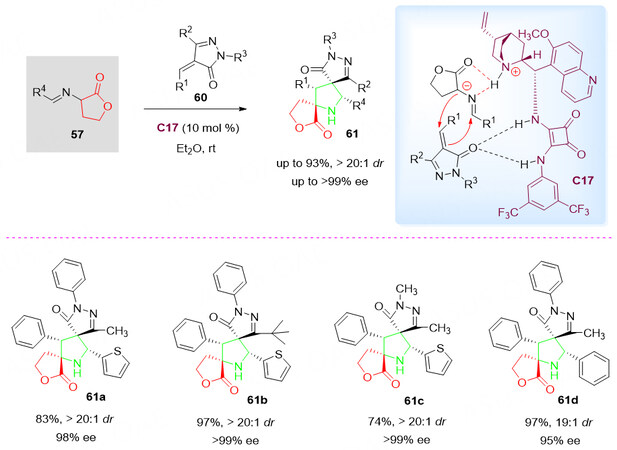
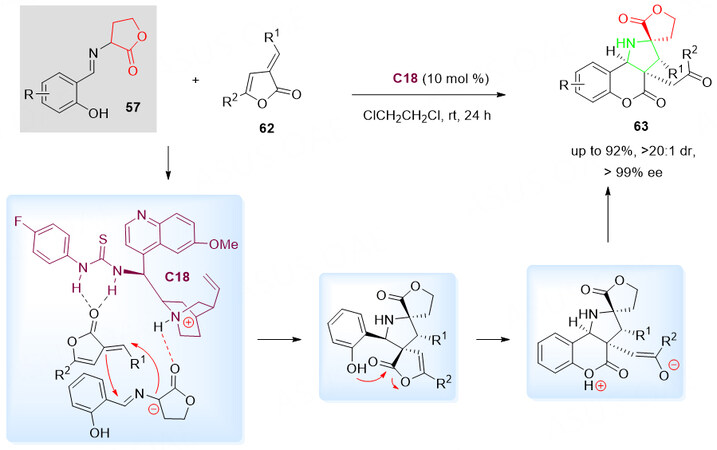
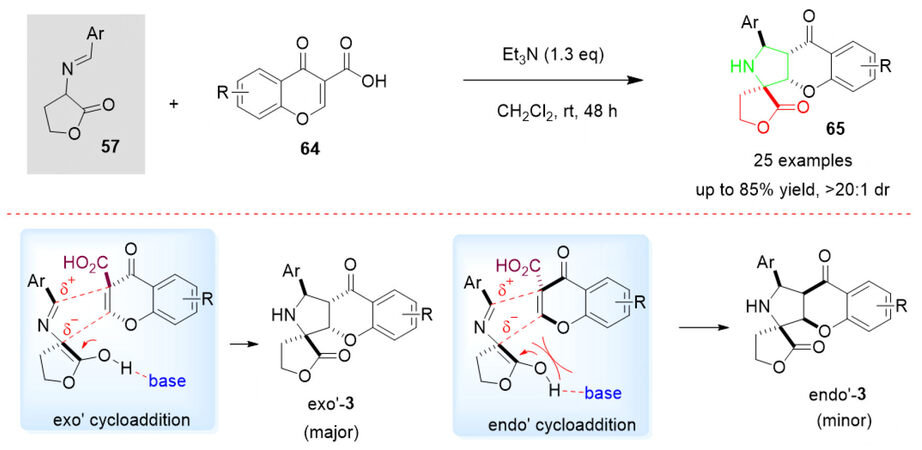
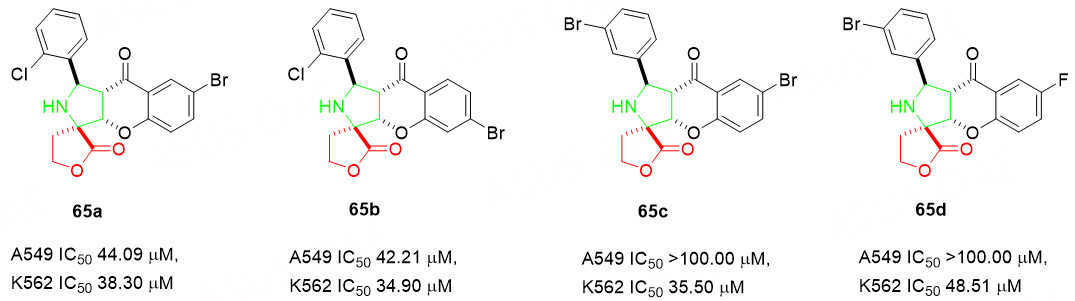
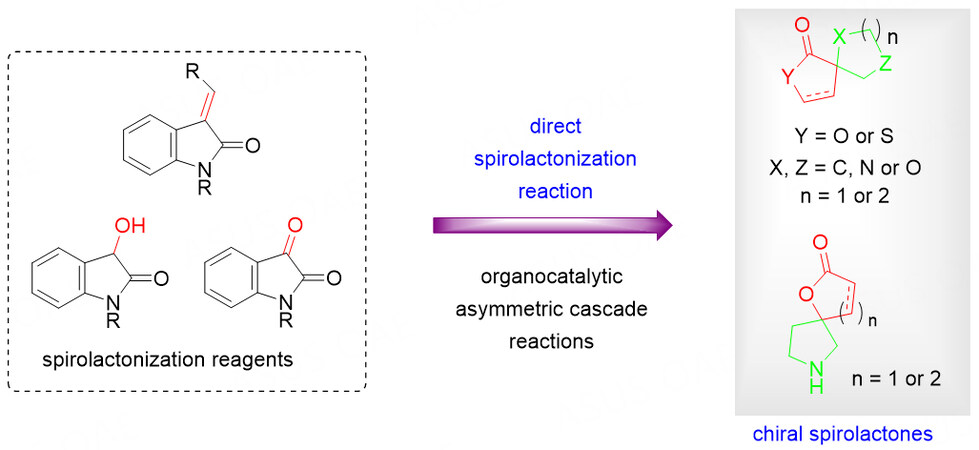
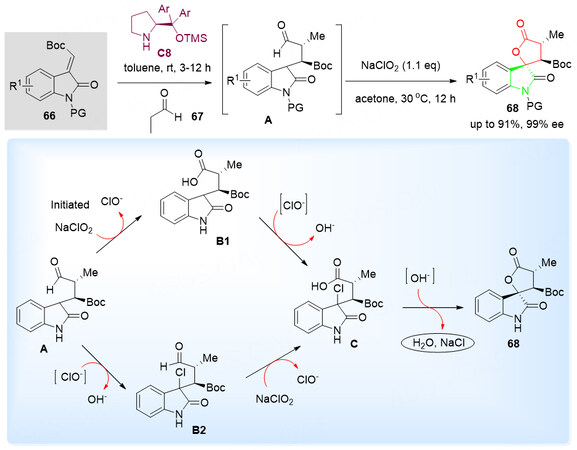
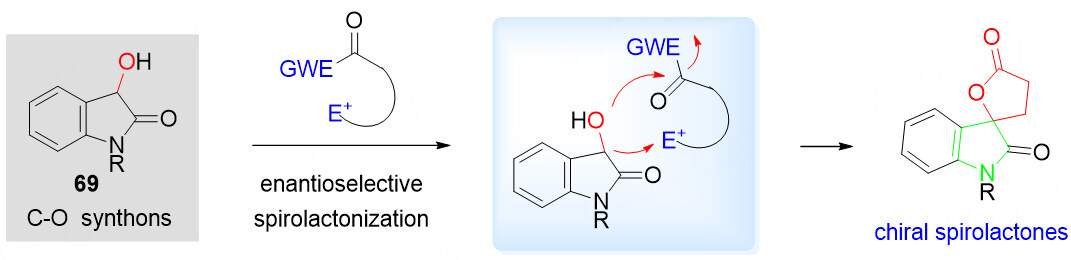
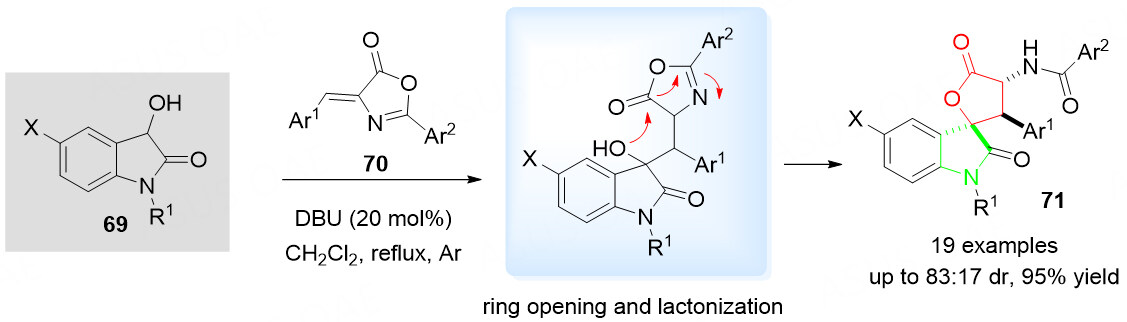
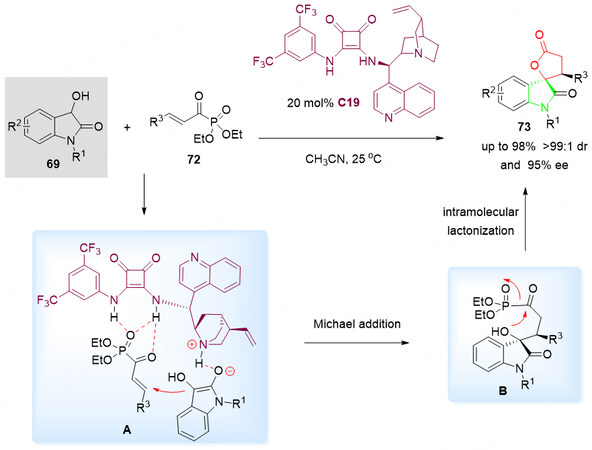
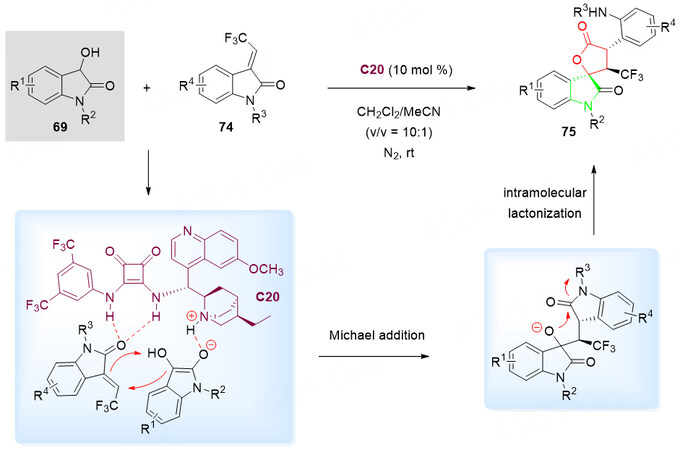

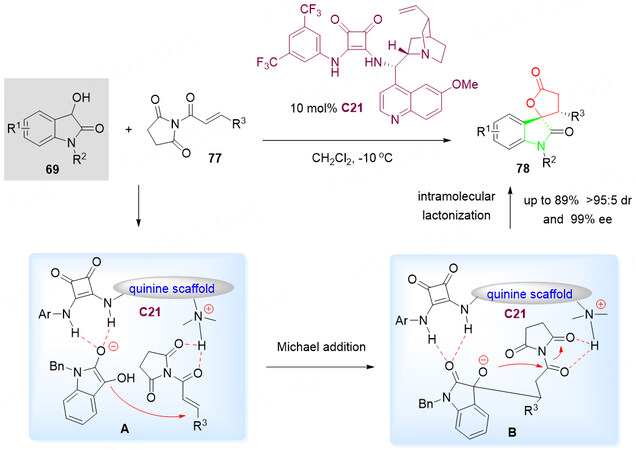
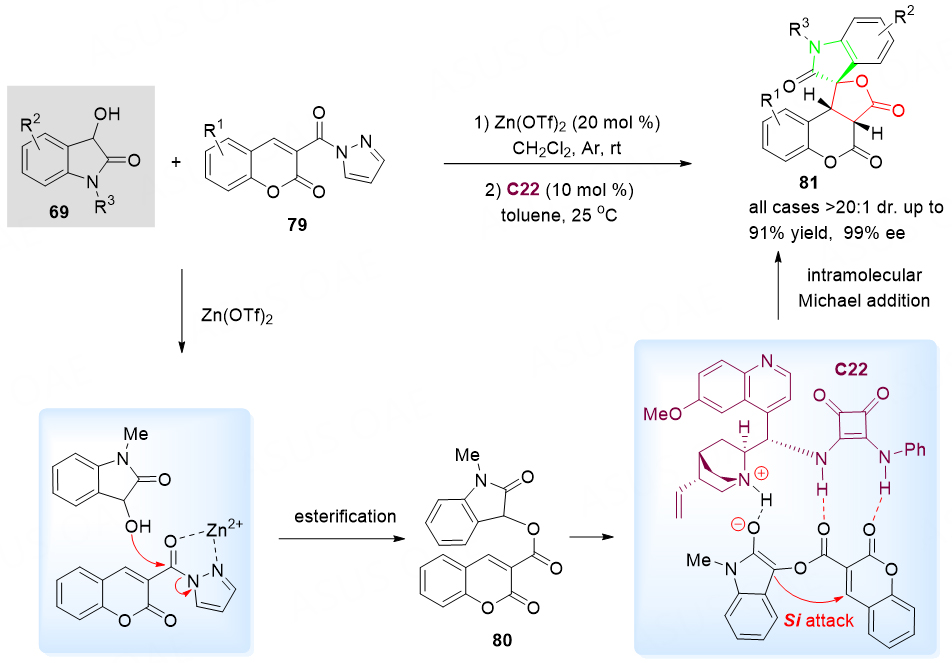
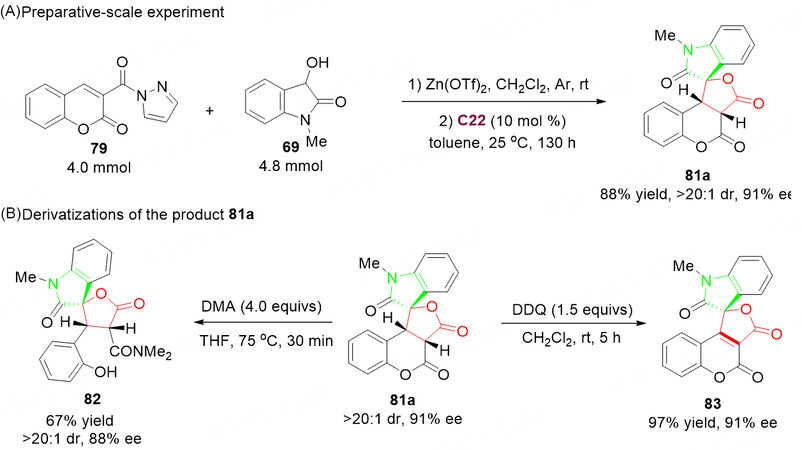
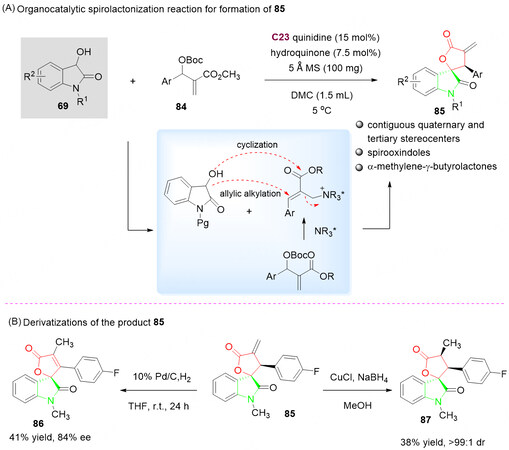
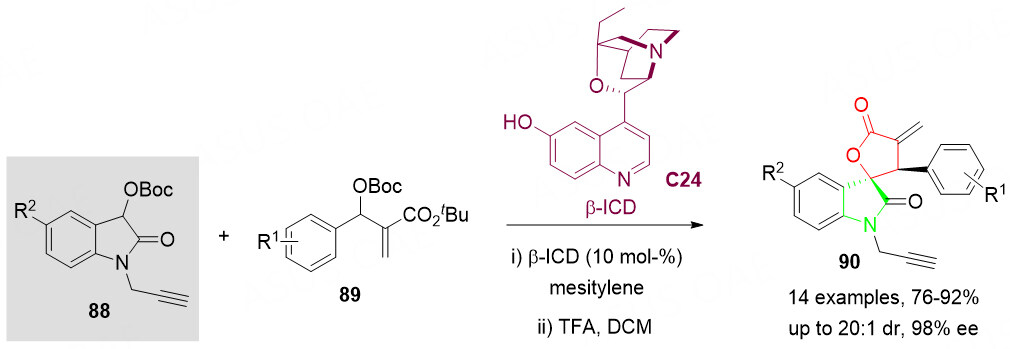
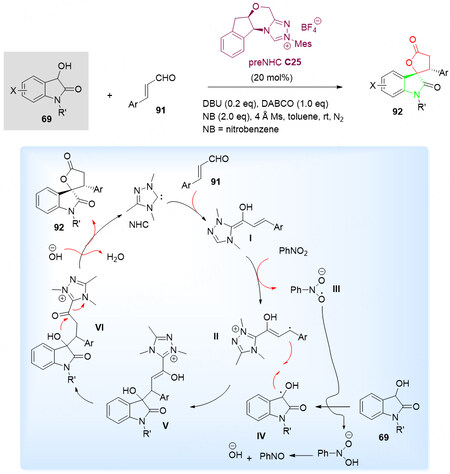
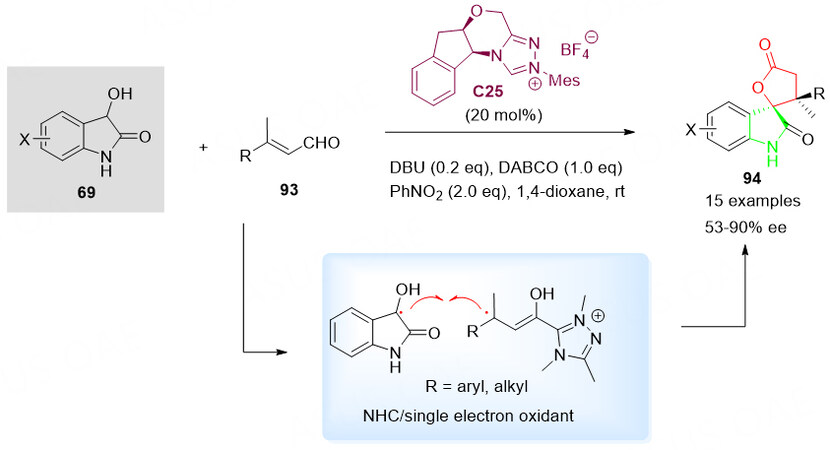
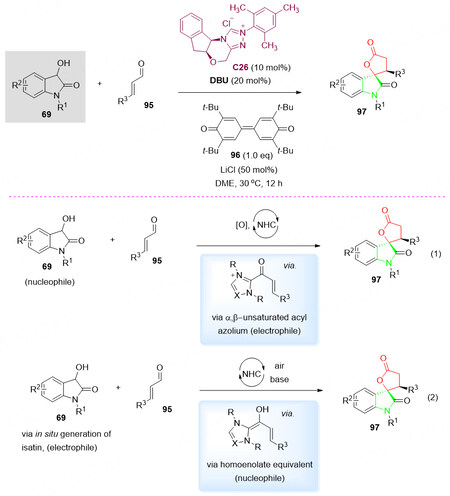
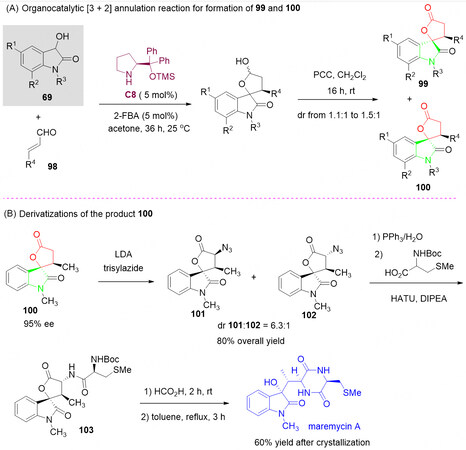
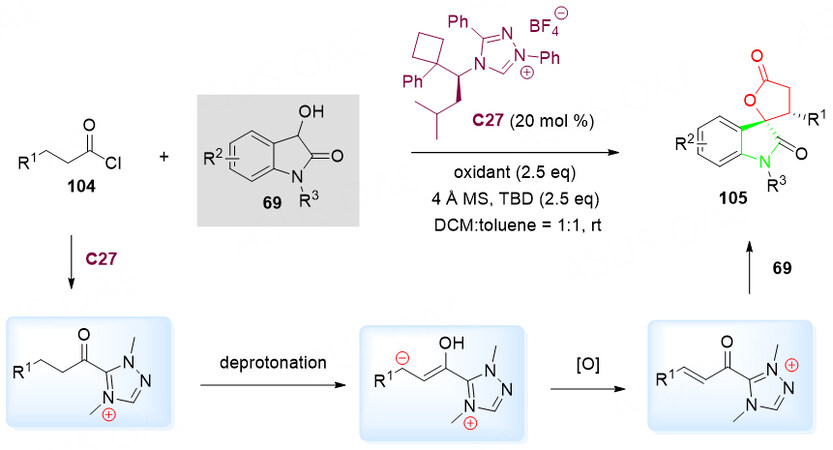
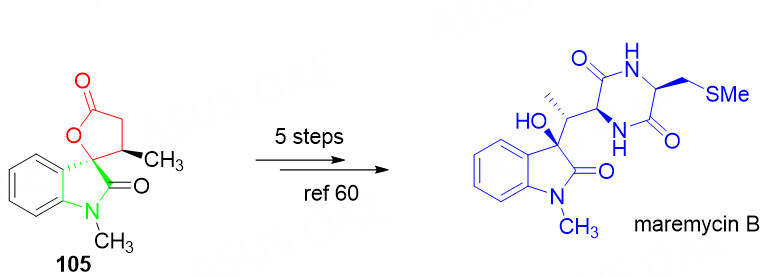
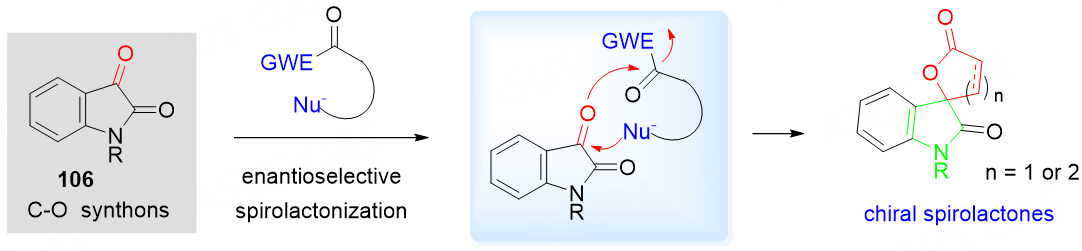
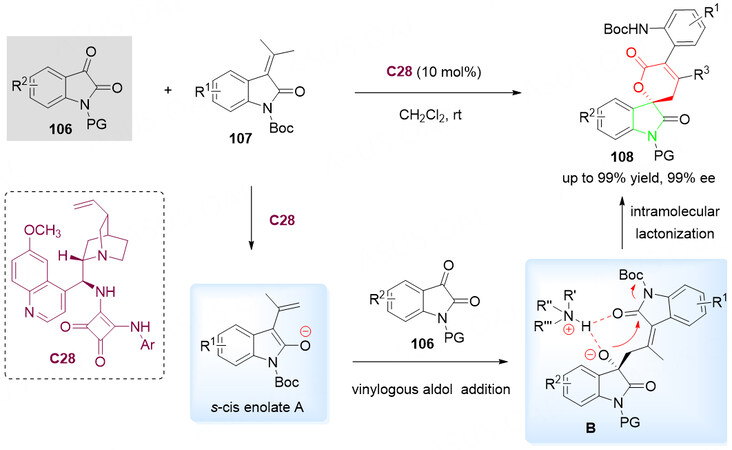
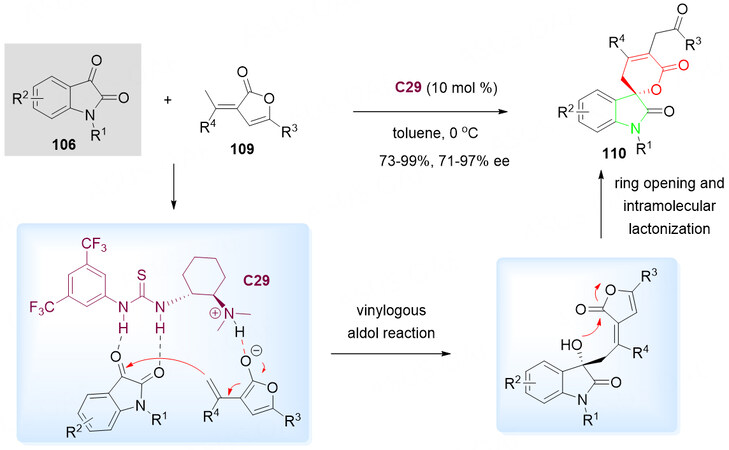
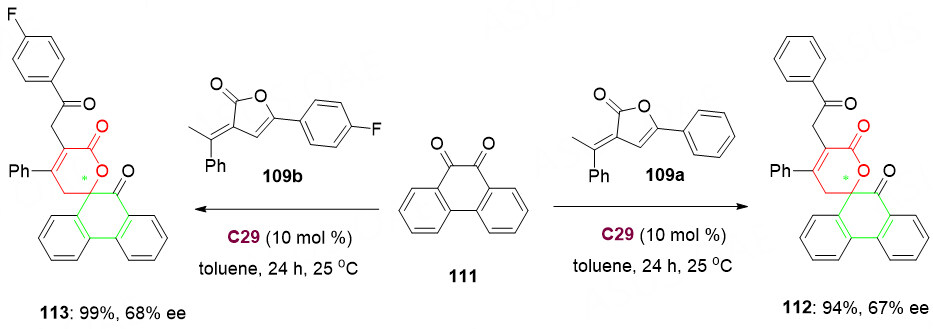
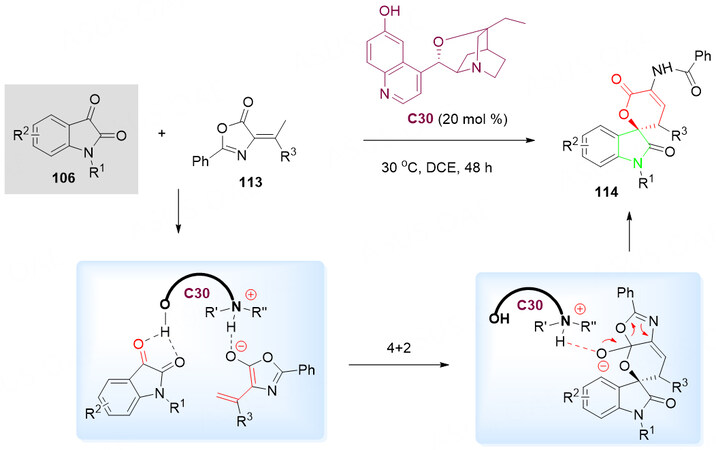
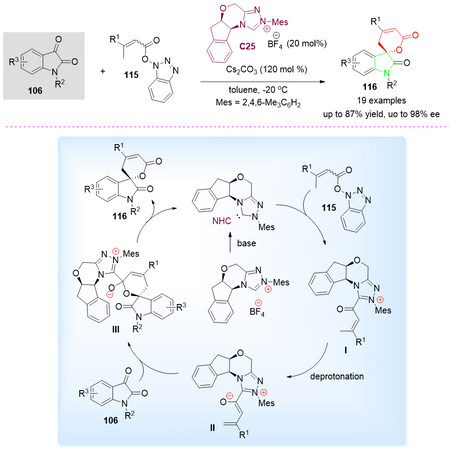
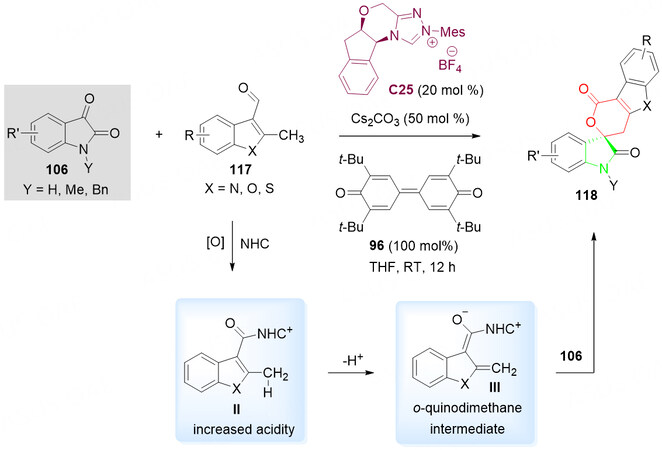
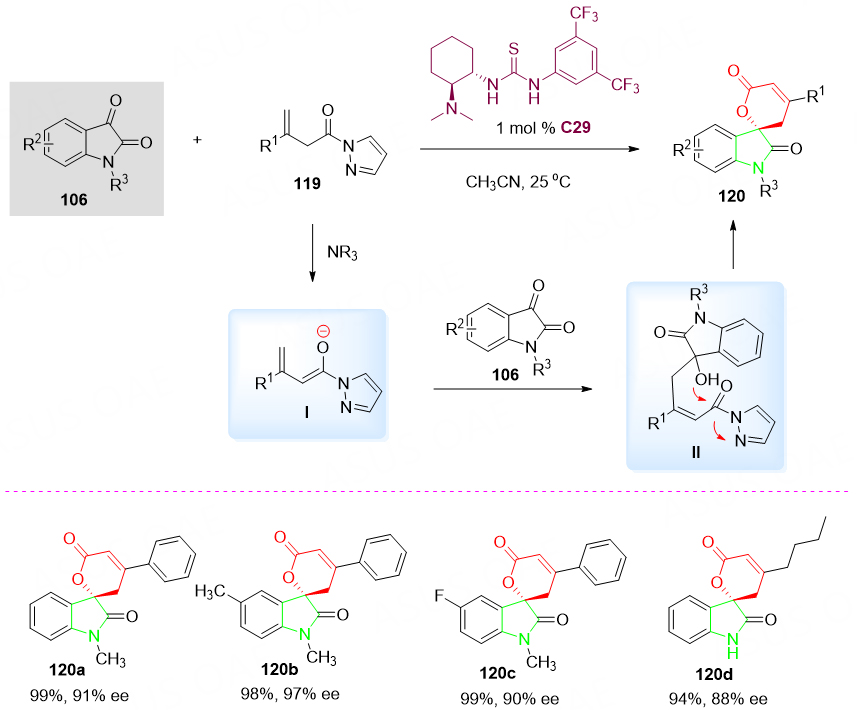
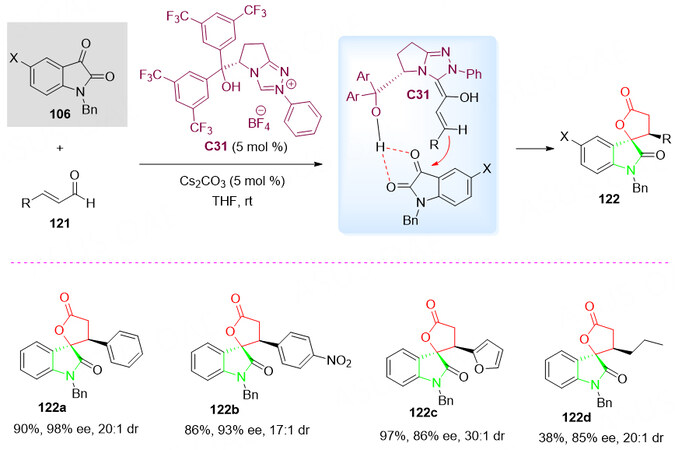
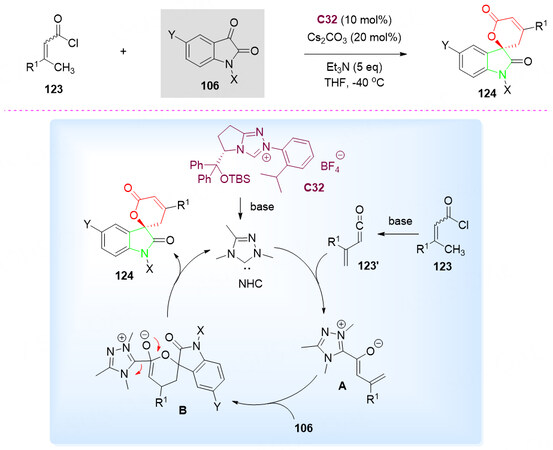
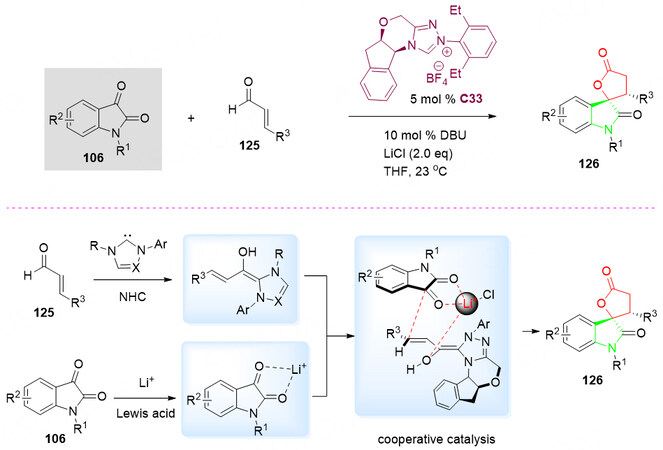
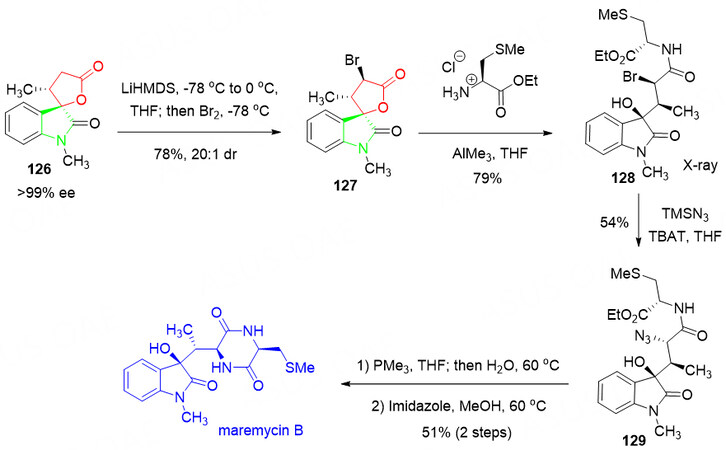
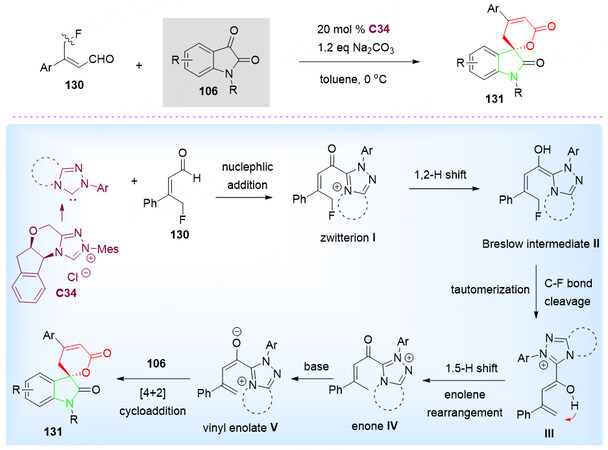
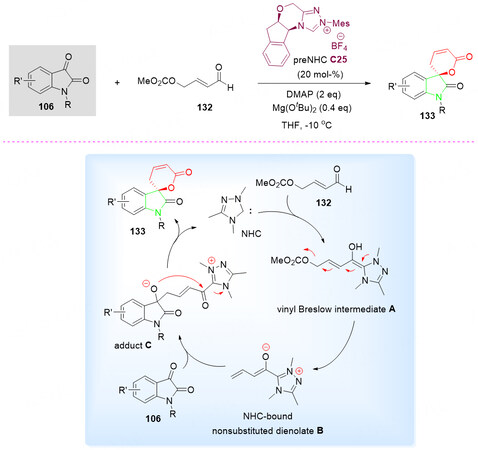











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.