Photothermal catalytic H2 production over hierarchical porous CaTiO3 with plasmonic gold nanoparticles
Abstract
The synergistic promotion by photocatalysis and thermocatalysis is a promising approach for sustainable hydrogen (H2) production. Herein, we rationally design a perovskite-based catalyst with three-dimensionally ordered macroporous structure (3DOM CaTiO3-Au) for photothermal catalytic H2 production from different substrates. The hierarchical 3DOM structure facilitates light harvesting and mass diffusion of the substrates, while the gold nanoparticles (Au NPs) promote charge separation. The photogenerated and hot electrons are oriented accumulated on the surface of Au NPs. The non-metallic gold species [Au(I)] show more activity for H2 evolution. As a result, 3DOM CaTiO3-Au exhibits excellent activity for H2 production from glycerol and other substrates with hydroxyl groups. The present work demonstrates a feasible approach to improve sustainable H2 production by rationally designing and fabricating efficient photothermal catalysts.
Keywords
INTRODUCTION
Hydrogen (H2), a clean and sustainable energy vector, has been widely recognized as a promising alternative to traditional fossil fuels[1]. Currently, about 96% of H2 is produced from the steam reforming process, which requires a huge amount of energy input and results in CO2 emission[2]. Comparably, solar-driven water splitting is a more energy-saving and sustainable approach for H2 generation[3-8]. However, the present water splitting activity has been greatly limited by the energetically and kinetically demanding process of O2 evolution reaction (OER)[9,10]. In the present work, numerous investigations have been conducted to replace the OER with organic photo-oxidation, which could not only reduce the energy demand for H2 generation but also provide an alternative to simultaneously produce value-added chemicals[11-17].
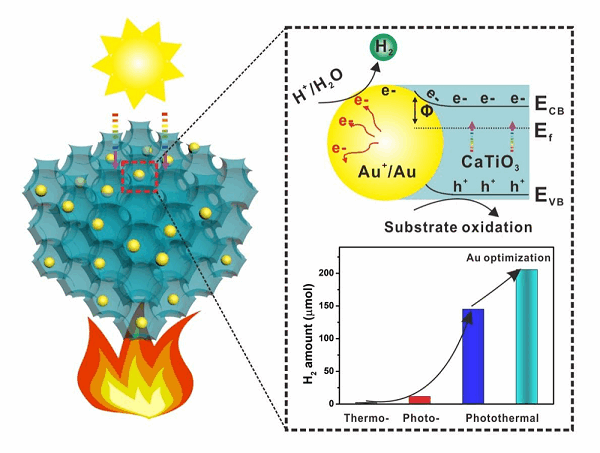
To realize the above scenario, the rational design of dual-functional photocatalysts is highly desired. The excellent candidate should have multiple abilities in light harvesting, enhanced charge separation and favorable surface reaction kinetics[18,19]. In terms of light harvesting, the hierarchically porous structure stands out due to the multiple scattering effect, prolonging the interaction between incident photons with photocatalytic materials[20-22]. Three-dimensionally ordered macroporous (3DOM) structure has been extensively applied in photocatalytic applications because of its outstanding competence in improving light absorption and facilitating mass diffusion[23-26]. Our previous investigation has revealed the significant promotion in photocatalytic H2 production by CaTiO3 perovskite with 3DOM structure and carbon quantum dots as cocatalysts, which exhibited comparable activity as 3DOM CaTiO3-Au[27]. Considering the excellent photo- and thermal effects of perovskite and gold nanoparticles, it could be foreseen that H2 production activity would be significantly boosted with the synergistic promotion by photocatalysis and thermocatalysis[28,29].
Limited by the high Gibbs free energy of most chemical reactions, the overall activity of most photocatalysis is still in the primary stage[30]. Herein, photothermal catalysis with the assistance of heating to improve the solar-to-hydrogen efficiency has begun to emerge in most recent years[31-33]. Metal nanomaterials with localized surface plasmon resonance (LSPR) effect have been widely utilized in photothermal applications, such as gold[34]. The widely recognized mechanism in this case is the photo-assisted thermocatalysis, in which the local temperature on these metal nanomaterials could be as high as > 70 °C under irradiation, thereby enhancing reaction kinetics[35]. However, the synergistic promotion of H2 generation could only achieve 2-5 folders as the LSPR of these metal nanomaterials generates a limited number of active electrons, which would be partially transferred to the conduction band of the contacted semiconductors[36]. Due to the sluggish H2 evolution reaction on the surface of semiconductor compared with metal materials, photo-assisted thermocatalysis still has significant scope for improvement. From this point of view, accumulating more electrons on the surface of plasmonic gold nanoparticles with extra heating from activated semiconductor materials is a feasible approach to further improve the activity of H2 production. Besides, non-metallic gold species (Auδ+) have been reported to have improved activity for photocatalytic reactions[37]. The internal mechanism has not been systematically investigated.
In the present work, we demonstrate the synergistic catalysis of photocatalysis and thermocatalysis for H2 production by conducting the experiment under light irradiation and at a controlled temperature. 3DOM CaTiO3 is firstly synthesized by the colloid-template method and gold nanoparticles (Au NPs) are then loaded on the surface to obtain the photothermal catalyst. The as-prepared 3DOM CaTiO3-Au exhibits hierarchically porous structure in long range and shows significant improvement in visible light absorption due to the LSPR effect. As a result, the 3DOM CaTiO3-Au shows excellent H2 production (145.2 μmol) from photothermal glycerol reforming, which is about 57 times and 13 times higher than that from thermocatalysis and photocatalysis, respectively. The oriented accumulation of photothermal electrons on the surface of Au NPs with oxidized species greatly reduces the energy barrier for hydrogen evolution. The present work demonstrates an example of boosting hydrogen production by coupling photocatalysis with thermocatalysis with the rational design of a catalyst.
RESULTS AND DISCUSSION
The fabrication process of 3DOM CaTiO3-Au is illustrated in Figure 1A. Briefly, the obtained polystyrene spheres by the surfactant-free emulsion polymerization method are assembled into a colloid template. The precursor containing Ca(NO3)2 and titanium (IV) isopropoxide then fills the voids of the template, accompanied by the simultaneous hydrolysis reaction. After calcination in air with gradient programs, the 3DOM CaTiO3 is obtained with CaCO3 impurity on the surface. Finally, gold nanoparticles are loaded on the acid-washed 3DOM CaTiO3 by sodium borohydride reduction method. As the quality of colloid template plays a vital role in the formation of 3DOM structure, the assembled polystyrene spheres are first characterized by scanning electron microscopy (SEM). The highly ordered assembly of polystyrene spheres is revealed in Figure 1B. Figure 1C displays the low-resolution SEM image of the fabricated 3DOM CaTiO3 which is an assembly of relatively uniform three-dimensionally ordered macroporous structure. The framework of 3DOM CaTiO3 is constructed by porous nanosheet, which has been well explained in our previous investigation [Figure 1D]. Transmission electron microscopy (TEM) images at low resolution also reveal the hierarchically porous structure with well-distributed gold nanoparticles [Figure 1E and F]. The statistical result of the gold nanoparticles shows a broad size distribution and they concentrate in 8 nm
Figure 1. (A) Schematic illustration of the fabrication process of 3DOM CaTiO3-Au, SEM images of (B) assembled polystyrene spheres; (C) 3DOM CaTiO3 with low resolution; (D) 3DOM CaTiO3 with high resolution; (E) and (F) Low-resolution TEM images of 3DOM CaTiO3-Au; (G) high-resolution TEM image of 3DOM CaTiO3-Au.
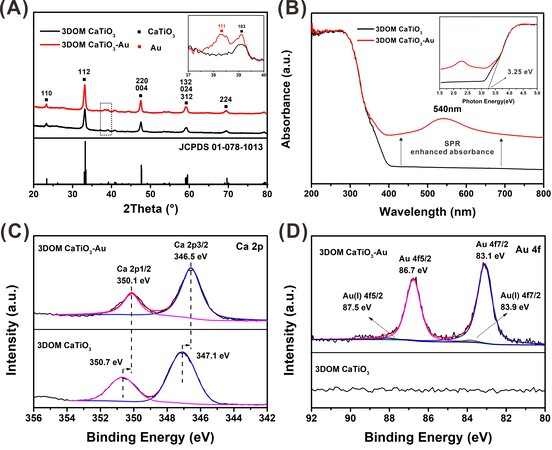
Figure 2A shows the XRD patterns of the 3DOM CaTiO3 and 3DOM CaTiO3-Au. CaTiO3 phase was confirmed by the comparison between the XRD patterns and the Joint Committee on Powder Diffraction Standards (JCPDS) card no. 01-078-1013. The as-fabricated CaTiO3 has pure phase and good crystallinity. All diffraction peaks can be assigned to the orthorhombic structure. The inset in Figure 2A is the partial enlarged detail and the peak around 38.3° corresponds to the (111) lattice plane of Au, which reveals the presence of Au in CaTiO3. The XRD patterns of the CaTiO3 unwashed by diluted nitric acid are shown in Supplementary Figure 3. In addition to CaTiO3, the impurity CaCO3 was also observed. The Ca2+ outside the crystal structure of CaTiO3 interacted with CO2 to form CaCO3 during the calcination process. The initial molar ratio of Ca and Ti was 1:1, and the formation of CaCO3 indicated the presence of cation defects in the crystal structure[38].
Figure 2. (A) XRD patterns; (B) UV-vis absorption spectra, high-resolution XPS spectra of (C) Ca 2p; and (D) Au 4f of 3DOM CaTiO3 and 3DOM CaTiO3-Au.
The optical properties of 3DOM CaTiO3 and 3DOM CaTiO3-Au have been measured by UV-vis diffuse reflectance spectroscopy and the corresponding spectra are shown in Figure 2B. The optical band gap values obtained through the linear fitting of tails from the UV-vis spectra are shown in the inset of Figure 2B. All the samples are of the same size and thickness. The strong absorption at a wavelength range below 400 nm matches the intrinsic inter-band transition absorption of orthorhombic CaTiO3[39]. As shown in the inset of Figure 2B, the band gap of as-fabricated 3DOM CaTiO3 is approximately 3.25 eV, corresponding to an optical absorption edge of 382 nm. After the introduction of AuNPs, the light absorption is greatly improved. The broad peak at 540 nm of 3DOM CaTiO3-Au composite corresponds to surface plasmon resonance (SPR) absorption of Au nanoparticles.
The chemical states on the surface of catalysts have been investigated by X-ray photoelectron spectroscopy (XPS), where characteristic peaks of Ca, Ti, O, Au and C are observed in the general survey scan
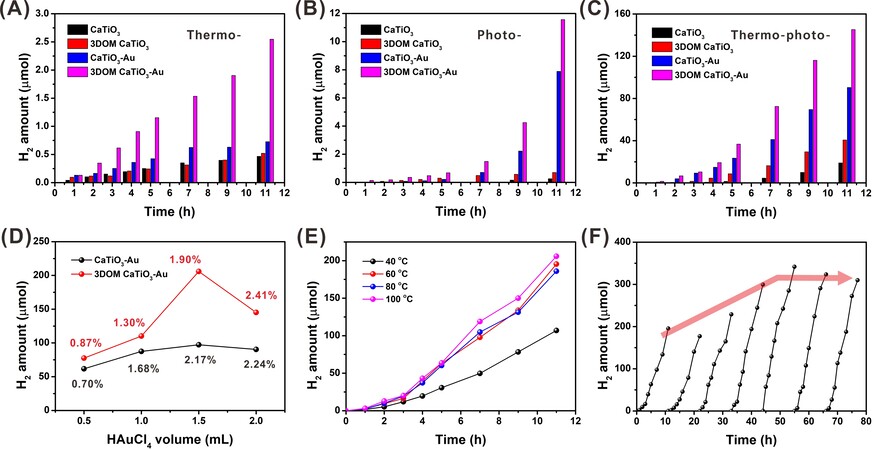
The activity of as-prepared catalysts was evaluated by thermos-photocatalytic glycerol reforming for H2 production. To reveal the synergistic promotion of thermocatalysis and photocatalysis, H2 production over different catalysts was first investigated via thermocatalysis at 100 °C and photocatalysis, respectively. CaTiO3 and 3DOM CaTiO3 produce a similar amount of H2 after 11 h reaction (0.47 μmol and 0.52 μmol respectively), indicating the negligible contribution of 3DOM structure in thermocatalytic H2 production of pristine CaTiO3 [Figure 3A]. With the loading of gold nanoparticles, the amount of H2 significantly increases to 2.55 μmol after 11 h reaction, which is probably attributed to the mass diffusion property of hierarchical 3DOM structure. Another feature of 3DOM structure is light harvesting, which facilitates activating CaTiO3 to produce more electrons and holes[41]. The promotion of H2 production of 3DOM structure could be revealed by the performance of CaTiO3 (0.26 μmol) and 3DOM CaTiO3 (0.70 μmol) under the Xenon lamp irradiation [Figure 3B]. After loading gold nanoparticles, about 30 folders and 17 folders in H2 production after 11 h reaction is achieved for CaTiO3-Au (7.89 μmol) and 3DOM CaTiO3-Au (11.67 μmol), respectively. The synergistic enhancement is further realized by coupling thermocatalysis at 100 °C and photocatalysis for all the four catalysts. The amount of H2 generation at 11-h reaction for
Figure 3. H2 production over different catalysts via (A) thermocatalysis (100 °C); (B) photocatalysis; (C) thermo-photocatalysis (100 °C); (D) thermo-photocatalytic H2 production over 3DOM CaTiO3-Au prepared by different volumes of HAuCl4 (actual loading of Au is labeled); (E) thermo-photocatalytic H2 production over 3DOM CaTiO3-Au prepared by 1.5 mL of HAuCl4 under different reaction temperatures; and (F) long-time cycling test.
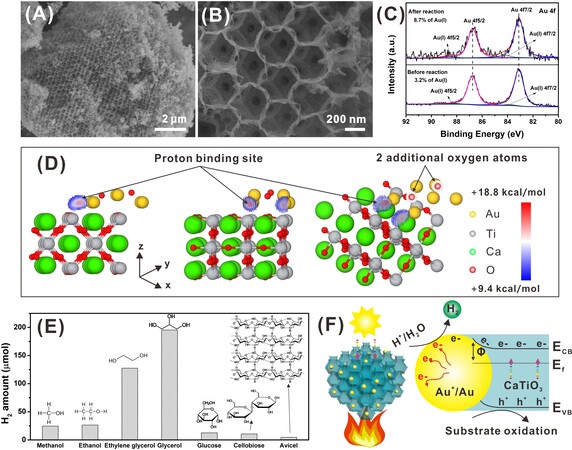
To explain the internal reason for the gradual enhancement in H2 production during the first five cycles, the used catalyst 3DOM CaTiO3-Au was collected and characterized for its morphology and chemical state. The 3DOM structure in the long range is kept well after catalytic reaction and a little collapse could also be observed in the high-resolution SEM image [Figure 4A and B]. A significant change in the chemical state of gold is observed [Figure 4C]. Other elements have almost the same chemical state after catalytic reaction
Figure 4. (A and B) SEM images of 3DOM CaTiO3-Au after photocatalytic reaction; (C) XPS Au 4f spectra of 3DOM CaTiO3-Au before and after photocatalytic reaction; (D) electrostatic potential difference brought by oxidization of gold atoms (the left and the middle are cross-section view of yz and xz plane, respectively. The right is a 3-D view of the electrostatic potential difference. The positive values indicate an elevated electrostatic potential by the addition of oxygen atoms); (E) H2 production from different substrates; and (F) the proposed mechanism.
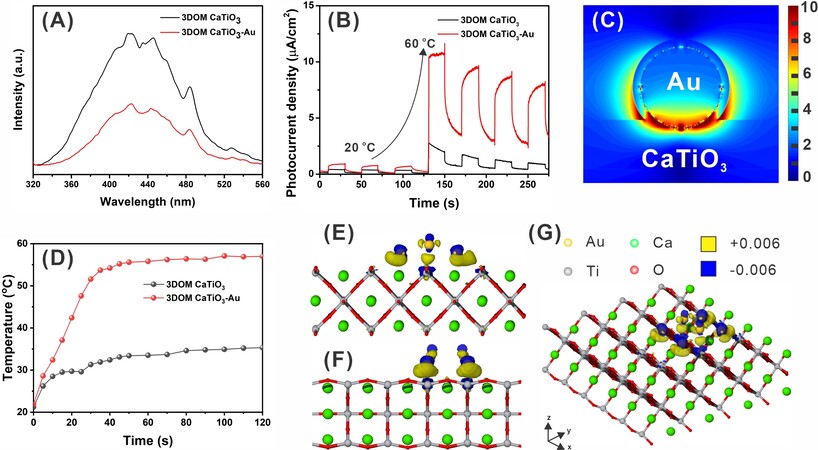
To reveal the improvement in charge separation after the introduction of Au NPs, steady photoluminescence (PL) spectra of 3DOM CaTiO3 and 3DOM CaTiO3-Au are collected with the excitation wavelength of 400 nm. 3DOM CaTiO3-Au exhibits much reduced PL intensity, indicating the significant inhibition of photogenerated electrons and holes [Figure 5A]. To check the effect of temperature on the charge generation, transient photocurrent is measured. Both 3DOM CaTiO3 and 3DOM CaTiO3-Au show excellent responses to light irradiation [Figure 5B]. When the temperature of the electrolyte increases from 20 °C to 60 °C, more than 10 folders in photocurrent are achieved for 3DOM CaTiO3-Au, revealing the generation of more electrons with the assistance of extra heating. To visually understand the charge separation, the electromagnetic field is simulated on a single Au nanoparticle [Figure 5C]. It is clear that the charge density on the surface of Au is much higher than that of CaTiO3 and the highest electromagnetic field intensity is located at the interface of Au and CaTiO3, indicating the formation of electron transfer channel at the interface and the immigration of active electrons from CaTiO3 to Au. The photothermal effect of the catalyst is recorded by measuring the temperature of the 3DOM CaTiO3 and 3DOM CaTiO3-Au with different light irradiation times [Figure 5D]. Combined with the corresponding IR thermal images
Figure 5. (A) PL; (B) photocurrent of 3DOM CaTiO3 and 3DOM CaTiO3-Au under different temperatures; (C) simulated electromagnetic field intensity distribution; (D) temperature of the catalyst with different light irradiation times for 3DOM CaTiO3 and 3DOM CaTiO3-Au. Isosurfaces of electronic density difference at the perovskite-gold interface from the cross-section view of (E) yz and (F) xz plane; (G)
CONCLUSIONS
In summary, 3DOM CaTiO3 is successfully fabricated by the colloid template method and Au NPs with partially oxidized species [Au(I)] are loaded to obtain 3DOM CaTiO3-Au as the efficient catalyst for photothermal H2 production. The 3DOM structure facilitates light harvesting and mass diffusion property, while the Au NPs help to improve the charge separation. As a result, 3DOM CaTiO3-Au with optimized Au loading exhibits boosted activity for H2 generation by the synergistic promotion of photocatalysis and thermocatalysis. Considerable H2 is also achieved when the substrates are changed into biomass derivates. The oriented accumulation of electrons on the surface of Au NPs and the presence of Au(I) species are revealed to be the key factors. The present work provides a rational catalyst design to boost hydrogen production through synergistic photocatalysis and thermocatalysis.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study, performed data analysis and interpretation, and wrote the draft of manuscript: Yu X
Performed data acquisition and provided administrative, technical, and material support: Zhao H
Performed DFT calculation: Yu Z
Discussed and revised the manuscript: Gates ID, Hu J
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was finacially supported by the Canada First Research Excellence Fund (CFREF).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Majumdar A, Deutch JM, Prasher RS, Griffin TP. A framework for a hydrogen economy. Joule 2021;5:1905-8.
2. Chen S, Pei C, Gong J. Insights into interface engineering in steam reforming reactions for hydrogen production. Energy Environ Sci 2019;12:3473-95.
3. Wang Z, Li C, Domen K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem Soc Rev 2019;48:2109-25.
4. Thalluri SM, Bai L, Lv C, Huang Z, Hu X, Liu L. Strategies for semiconductor/electrocatalyst coupling toward solar-driven water splitting. Adv Sci (Weinh) 2020;7:1902102.
5. Wang Y, Vogel A, Sachs M, et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat Energy 2019;4:746-60.
6. Zhang P, Tian Z, Kang Y, et al. 10 nm Corrugated TiO2 nanowire arrays by monomicelle-directed assembly for efficient hole extraction. J Am Chem Soc 2022;144:20964-74.
7. Gao M, Zhang T, Ho GW. Advances of photothermal chemistry in photocatalysis, thermocatalysis, and synergetic photothermocatalysis for solar-to-fuel generation. Nano Res 2022;15:9985-10005.
8. Gao M, Peh CK, Zhu L, Yilmaz G, Ho GW. Photothermal catalytic gel featuring spectral and thermal management for parallel freshwater and hydrogen production. Adv Energy Mater 2020;10:2000925.
9. Jamesh M, Harb M. Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting - a review. J Energy Chem 2021;56:299-342.
10. Liu Y, Luo X, Zhou C, et al. A modulated electronic state strategy designed to integrate active HER and OER components as hybrid heterostructures for efficient overall water splitting. Appl Catal B Environ 2020;260:118197.
11. Uekert T, Pichler CM, Schubert T, Reisner E. Solar-driven reforming of solid waste for a sustainable future. Nat Sustain 2021;4:383-91.
12. Zhao H, Li CF, Liu LY, et al. n-p Heterojunction of TiO2-NiO core-shell structure for efficient hydrogen generation and lignin photoreforming. J Colloid Interf Sci 2021;585:694-704.
13. Zhao H, Li CF, Yong X, et al. Coproduction of hydrogen and lactic acid from glucose photocatalysis on band-engineered Zn1-xCdxS homojunction. iScience 2021;24:102109.
14. Nwosu U, Wang A, Palma B, et al. Selective biomass photoreforming for valuable chemicals and fuels: a critical review. Renew Sust Energ Rev 2021;148:111266.
15. Wang J, Kumar P, Zhao H, Kibria MG, Hu J. Polymeric carbon nitride-based photocatalysts for photoreforming of biomass derivatives. Green Chem 2021;23:7435-57.
16. Wu X, Luo N, Xie S, et al. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem Soc Rev 2020;49:6198-223.
17. Li Y, Zhang D, Qiao W, et al. Nanostructured heterogeneous photocatalyst materials for green synthesis of valuable chemicals. Chem Synth 2022;2:9.
18. Toe CY, Tsounis C, Zhang J, et al. Advancing photoreforming of organics: highlights on photocatalyst and system designs for selective oxidation reactions. Energy Environ Sci 2021;14:1140-75.
19. Ma J, Liu K, Yang X, et al. Recent advances and challenges in photoreforming of biomass-derived feedstocks into hydrogen, biofuels, or chemicals by using functional carbon nitride photocatalysts. ChemSusChem 2021;14:4903-22.
20. Chen LH, Li Y, Su BL. Hierarchy in materials for maximized efficiency. Natl Sci Rev 2020;7:1626-30.
21. Liu J, Zhao H, Wu M, et al. Slow photons for photocatalysis and photovoltaics. Adv Mater 2017;29:1605349.
23. Zhao H, Wu M, Liu J, Deng Z, Li Y, Su B. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl Catal B Environ 2016;184:182-90.
24. Zhao H, Yu X, Hu G, et al. Confined synthesis of BiVO4 nanodot and ZnO cluster co-decorated 3DOM TiO2 for formic acid production from the xylan-based hemicellulose photorefinery. Green Chem 2021;23:8124-30.
25. Zhao H, Li C, Yu X, et al. Mechanistic understanding of cellulose β-1,4-glycosidic cleavage via photocatalysis. Appl Catal B Environ 2022;302:120872.
26. Liu J, Guo Y, Hu Z, et al. Slow photon-enhanced heterojunction accelerates photocatalytic hydrogen evolution reaction to unprecedented rates. CCS Chem 2022:1-13.
27. Zhao H, Liu J, Li C, et al. Meso-microporous nanosheet-constructed 3dom perovskites for remarkable photocatalytic hydrogen production. Adv Funct Mater 2022;32:2112831.
28. Mateo D, Morlanes N, Maity P, Shterk G, Mohammed OF, Gascon J. Efficient visible-light driven photothermal conversion of CO2 to methane by nickel nanoparticles supported on barium titanate. Adv Funct Mater 2021;31:2008244.
29. Song C, Wang Z, Yin Z, Xiao D, Ma D. Principles and applications of photothermal catalysis. Chem Catal 2022;2:52-83.
30. Li Y, Xue J, Shen Q, et al. Construction of a ternary spatial junction in yolk–shell nanoreactor for efficient photo-thermal catalytic hydrogen generation. Chem Eng J 2021;423:130188.
31. Li X, Zhao S, Duan X, et al. Coupling hydrothermal and photothermal single-atom catalysis toward excellent water splitting to hydrogen. Appl Catal B Environ 2021;283:119660.
32. Gao M, Zhu L, Peh CK, Ho GW. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ Sci 2019;12:841-64.
33. Zhang T, Meng F, Gao M, et al. Multi-interfacial catalyst with spatially defined redox reactions for enhanced pure water photothermal hydrogen production. EcoMat 2021:3.
34. Liu H, Shi L, Zhang Q, et al. Photothermal catalysts for hydrogenation reactions. Chem Commun (Camb) 2021;57:1279-94.
35. Zhou L, Swearer DF, Zhang C, et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018;362:69-72.
36. Ng SWL, Gao M, Lu W, Hong M, Ho GW. Selective wavelength enhanced photochemical and photothermal H2 generation of classical oxide supported metal catalyst. Adv Funct Mater 2021;31:2104750.
37. Zhao H, Liu P, Wu X, et al. Plasmon enhanced glucose photoreforming for arabinose and gas fuel co-production over 3DOM TiO2-Au. Appl Catal B Environ 2021;291:120055.
38. Wegmann M, Watson L, Hendry A. XPS analysis of submicrometer barium titanate powder. J Am Ceram Soc 2004;87:371-7.
39. Cai J, Cao A, Huang J, et al. Understanding oxygen vacancies in disorder-engineered surface and subsurface of CaTiO3 nanosheets on photocatalytic hydrogen evolution. Appl Catal B Environ 2020;267:118378.
40. Zhu C, Wang AL, Xiao W, et al. In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv Mater 2018;30:e1705516.
41. Chang Y, Yu K, Zhang C, et al. Ternary CdS/Au/3DOM-SrTiO3 composites with synergistic enhancement for hydrogen production from visible-light photocatalytic water splitting. Appl Catal B Environ 2017;215:74-84.
42. Wu X, Zhao H, Khan MA, et al. Sunlight-driven biomass photorefinery for coproduction of sustainable hydrogen and value-added biochemicals. ACS Sustain Chem Eng 2020;8:15772-81.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yu X, Yu Z, Zhao H, Gates ID, Hu J. Photothermal catalytic H2 production over hierarchical porous CaTiO3 with plasmonic gold nanoparticles. Chem Synth 2023;3:3. http://dx.doi.org/10.20517/cs.2022.30
AMA Style
Yu X, Yu Z, Zhao H, Gates ID, Hu J. Photothermal catalytic H2 production over hierarchical porous CaTiO3 with plasmonic gold nanoparticles. Chemical Synthesis. 2023; 3(1): 3. http://dx.doi.org/10.20517/cs.2022.30
Chicago/Turabian Style
Yu, Xinti, Zechuan Yu, Heng Zhao, Ian D. Gates, Jinguang Hu. 2023. "Photothermal catalytic H2 production over hierarchical porous CaTiO3 with plasmonic gold nanoparticles" Chemical Synthesis. 3, no.1: 3. http://dx.doi.org/10.20517/cs.2022.30
ACS Style
Yu, X.; Yu Z.; Zhao H.; Gates ID.; Hu J. Photothermal catalytic H2 production over hierarchical porous CaTiO3 with plasmonic gold nanoparticles. Chem. Synth. 2023, 3, 3. http://dx.doi.org/10.20517/cs.2022.30
About This Article
Copyright
Author Biographies




His current research interests are in heavy oil and oil sands recovery process design and optimization, thermal (SAGD and CSS) and thermal-solvent (ES-SAGD, SA-CSS) oil recovery processes, cold production of heavy oil with sand (CHOPS) and follow-up process design, reservoir engineering and simulation, reservoir process optimization, reactive reservoir processes and simulation, heat and mass transfer, fluid mechanics, biofilm evolution in porous media, and bioreactor design (think of the reservoir as a bioreactor).

Data & Comments
Data
 Cite This Article 27 clicks
Cite This Article 27 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.