Regioselectivity of N-heteroarene electrocarboxylations: divided vs. undivided cell
Keywords
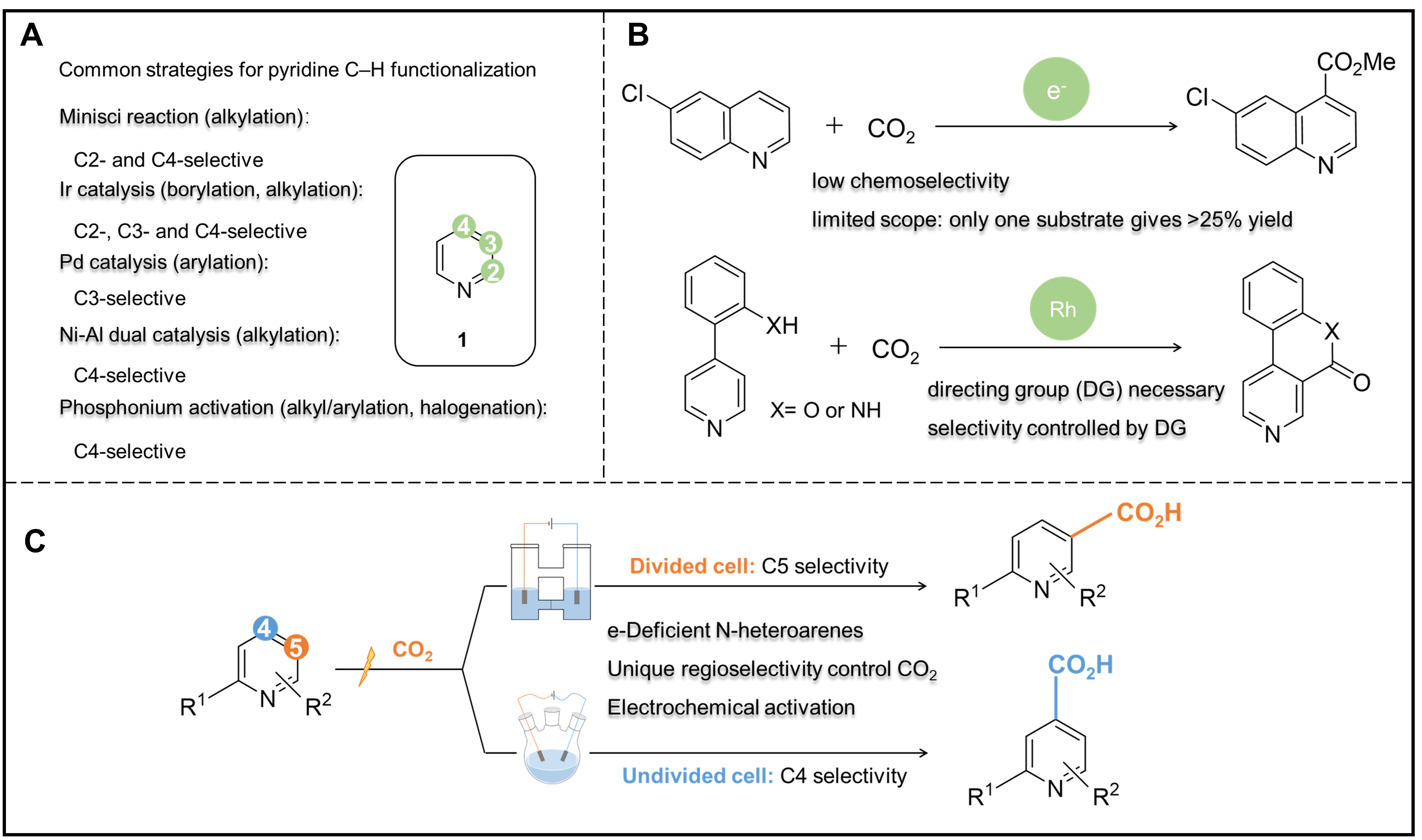
Site-selective C-H functionalized reactions of N-heteroarenes are effective approaches to the synthesis and modification of important molecules, thus providing great value for both academia and the pharmaceutical industry[1,2]. Among them, the CO2-participated C-H carboxylation of N-heteroarenes attracts great attention for both economic and sustainable purposes because it can directly upgrade the greenhouse gas CO2 to value-added molecule products[3,4]. Unfortunately, unlike the available transformation for arylation, alkylation, and borylation, the limited carboxylation synthetic methods so far still cannot break the intrinsic selectivity determined by the electro and steric structure of N-heteroarenes [Figure 1A and B][5-9]. How to realize the precise regioselective control of carboxylation remains a key challenge in this field.
Figure 1. Strategies for pyridine C-H functionalization. (A) Common strategies on different sites with alkylation, arylation, borylation and halogenation. (B) Precedents for C-H carboxylation of pyridine and related N-heteroarenes with CO2. (C) New concept of regiodivergent electrochemical C-H carboxylation of pyridines.
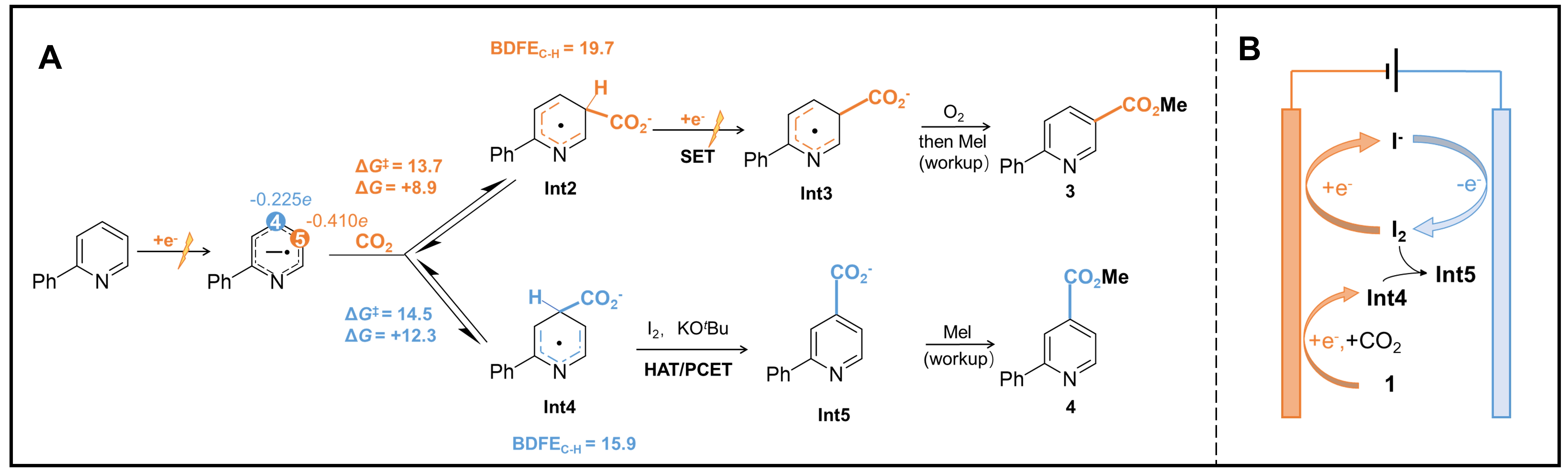
Electrochemical organic synthesis has gained significant attention for its ability to activate molecules into unusual intermediates by providing electrons or protons under controllable potential, which has also been recently adopted in the challenging oxidative functionalization of C-H bonds[10]. Prof. Da-Gang Yu and his colleagues, at Sichuan University, in collaboration with Prof. Song Lin at Cornell University, have adopted the electrochemical strategy to realize the direct carboxylation of N-heteroarenes using CO2[11]. Impressively, they found that by changing the type of the electrolytic cell, the regioselectivity could be finely tuned between C5-carboxylation (in a divided cell) and C4-carboxylation (in an undivided cell), as shown in Figure 1C. Further mechanistic studies suggest that the C5-carboxylation in the divided cell starts with one electron reduction of the pyridine ring, forming radical anion Int1 [Figure 2A]. Then, the nucleophilic addition of Int1 to CO2 takes place at the C5 position, which possesses the highest electron population among the pyridine ring carbon atoms. The resulting Int2 would be further cathodically reduced to dianion Int3, followed by oxidative rearomatization by O2 to C5-carboxylation product. However, DFT calculation depicts that the nucleophilic addition to CO2 on Int1 is reversible and endergonic on either C5 or C4 positions, allowing the carboxylation regioselectivity to be potentially altered if the follow-up reaction step favored an alternative pathway. Moreover, the calculation also reveals that if CO2 is added to the C4 position, the resulting Int4 may cost significantly lower energy during the following C-H dissociation step in the presence of a hydrogen-atom acceptor. Therefore, when the reaction takes place in an undivided cell, the anodically generated hydrogen acceptor I2 from the electrolyte nBu4NI promotes the more thermodynamically-favored conversion from Int4 to Int5 through either direct hydrogen-atom transfer (HAT) or proton-coupled electron transfer (PCET) process, and eventually diverts the reaction to
Figure 2. Possible mechanism diagram of the CO2-participated C-H carboxylation of N-heteroarenes. (A) Reaction pathways determined by different mechanisms: C5-carboxylation via electro-reductive activation and C4-carboxylation via paired-electrolysis-enabled HAT. (B) Achievement of C4-carboxylation and regeneration of hydrogen acceptors in the undivided cell.
More control experiments are conducted to verify their mechanism. By replacing nBu4NI with other electrolytes, the C4-carboxylation is inhibited mainly due to the failure of generating hydrogen acceptor at the anodic side, while the reactivity of C5-carboxylation stays unaffected. The importance of pairing both electrodes in the C4-carboxylation is further highlighted by carrying an alternating current (AC) electrolysis in a divided cell and discovering C4-carboxylation as the major pathway. Moreover, it was later found that this method could be expanded to more substrates including bi- and terpyridines, pyrimidines, pyrazines and quinolines.
Meanwhile, Dr. Zhao et al. also observed the C4-carboxylation of pyridines during their work on C-H carboxylation of (hetero-)arenes under a similar condition using iodide-containing electrolytes in an undivided cell[12]. Interestingly, although the influence of the cell type was not explored, they discovered that the electrochemical carboxylation method could be expanded to substrates beyond pyridines. This included electron-deficient naphthalenes, simple phenyl derivatives, and substituted quinolines, all with high regioselectivity. This finding raises the question of whether the electrolyte cell type could similarly impact the electrocarboxylation regioselectivity of other arene-based substrates, and if so, whether this would result in differences in the underlying mechanism.
In summary, this work by Yu and Lin provides an effective strategy for the regioselective C-H functionalization of pyridines and related N-heteroarenes with CO2 bringing advantages such as mild reaction conditions and wide substrate compatibility. Their discovery promotes the development of pharmacy and agronomy and facilitates biomolecular progress by not only enhancing the mechanistic understanding of the C-H carboxylation reaction but also contributing to the utilization of CO2.
DECLARATIONS
AcknowledgmentsWe sincerely thank all leading chemists and co-workers involved in the development of the C-H functionalization of N-heteroarenes.
Authors’ contributionsWrote the draft manuscript: Zhong G
Revised and rewrote the manuscript: Huang Y, He L
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis study was financially supported by the National Natural Science Foundation of China (22202222).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Ye JH, Ju T, Huang H, Liao LL, Yu DG. Radical carboxylative cyclizations and carboxylations with CO2. Acc Chem Res 2021;54:2518-31.
2. Murakami K, Yamada S, Kaneda T, Itami K. C-H functionalization of azines. Chem Rev 2017;117:9302-32.
3. Tortajada A, Juliá-Hernández F, Börjesson M, Moragas T, Martin R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew Chem Int Ed Engl 2018;57:15948-82.
4. Fosu SC, Hambira CM, Chen AD, Fuchs JR, Nagib DA. Site-selective C-H functionalization of (Hetero)arenes via transient, non-symmetric iodanes. Chem 2019;5:417-28.
5. Cheng C, Hartwig JF. Rhodium-catalyzed intermolecular C-H silylation of arenes with high steric regiocontrol. Science 2014;343:853-7.
6. Liu Q, Wu L, Jackstell R, Beller M. Using carbon dioxide as a building block in organic synthesis. Nat Commun 2015;6:5933.
7. Proctor RSJ, Phipps RJ. Recent advances in Minisci-type reactions. Angew Chem Int Ed Engl 2019;58:13666-99.
8. Ye M, Gao GL, Yu JQ. Ligand-promoted C-3 selective C-H olefination of pyridines with Pd catalysts. J Am Chem Soc 2011;133:6964-7.
9. Kim JH, Constantin T, Simonetti M, Llaveria J, Sheikh NS, Leonori D. A radical approach for the selective C-H borylation of azines. Nature 2021;595:677-83.
10. Fuchs P, Hess U, Holst H, Lund H, Enzell CR. Electrochemical carboxylation of some heteroaromatic compounds. Acta Chem Scand B 1982;13:185-92.
11. Sun GQ, Yu P, Zhang W, et al. Electrochemical reactor dictates site selectivity in
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhong G, Huang Y, He L. Regioselectivity of N-heteroarene electrocarboxylations: divided vs. undivided cell. Chem Synth 2023;3:19. http://dx.doi.org/10.20517/cs.2023.03
AMA Style
Zhong G, Huang Y, He L. Regioselectivity of N-heteroarene electrocarboxylations: divided vs. undivided cell. Chemical Synthesis. 2023; 3(3): 19. http://dx.doi.org/10.20517/cs.2023.03
Chicago/Turabian Style
Zhong, Gang, Yang Huang, Lin He. 2023. "Regioselectivity of N-heteroarene electrocarboxylations: divided vs. undivided cell" Chemical Synthesis. 3, no.3: 19. http://dx.doi.org/10.20517/cs.2023.03
ACS Style
Zhong, G.; Huang Y.; He L. Regioselectivity of N-heteroarene electrocarboxylations: divided vs. undivided cell. Chem. Synth. 2023, 3, 19. http://dx.doi.org/10.20517/cs.2023.03
About This Article
Copyright
Data & Comments
Data
 Cite This Article 19 clicks
Cite This Article 19 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.