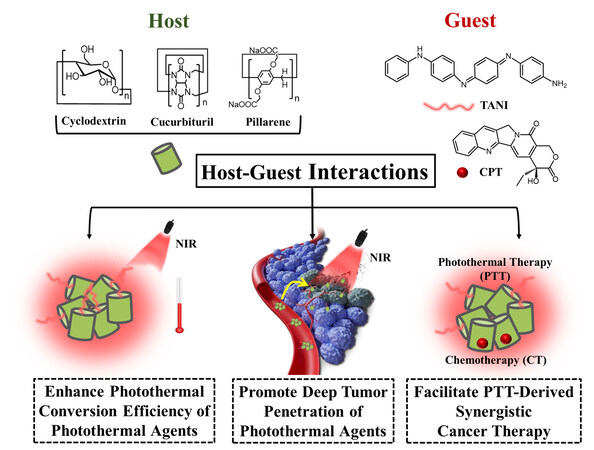
Host-guest assemblies for improved photothermal cancer therapy
Abstract
Photothermal cancer therapy has attracted plenty of attention in the last decades due to its promising efficacy, spatiotemporal control, negligible drug resistance, etc. However, to achieve widespread clinical application, studies in the field of photothermal therapy still need to focus on the improvement of efficacy and the minimization of side effects. Host-guest assemblies, constructed by the inclusion of small molecular guests into macrocyclic hosts through non-covalent interactions, are featured with unique microenvironments and flexible, dynamic nature. Based on the abovementioned advantages, host-guest assemblies show great potential in photothermal therapy. However, presumably, the endeavors of host-guest assemblies-based photothermal therapy have not been systematically discussed. Hence, to benefit the design of advanced host-guest assembly-based photothermal agents and promote the development of photothermal therapy, in this review, the major achievements of host-guest assemblies in photothermal cancer therapy, including the enhancement of photothermal conversion efficiency, the improvement of targeted distribution at tumor sites, and the superiority of constructing photothermal therapy-derived multimodal synergistic therapy, are discussed. In addition, the future perspectives on host-guest assemblies-based photothermal therapy are outlined.
Keywords
INTRODUCTION
Cancer, as one of the most malignant diseases, has become a serious threat to global public health[1,2]. Currently, the common clinical modalities of cancer treatment are surgery, chemotherapy and radiotherapy. Though demonstrated to be effective, all of the above methods have drawbacks[3-6]. Surgery can cause unpleasant pain to patients and unavoidably, induce cancer metastasis[7]. Chemotherapy and radiotherapy are well known to have side effects and, additionally, cause undesired drug resistance[8]. Therefore, the development of novel approaches to cancer treatment is still urgent.
Photothermal cancer therapy is a therapeutic modality that converts near-infrared (NIR) photon energy into heat and, subsequently, initiates the apoptosis of cancer cells through hyperthermia[9-11]. Due to the high selectivity, spatiotemporal control, negligible drug resistance, etc.[12-14], photothermal therapy has attracted much attention in the last few decades and is regarded as a promising approach to cancer treatment. In order for photothermal therapy to achieve satisfactory therapeutic effects and low side effects, several factors need to be taken into consideration for the design of photothermal agents. Firstly, high photothermal conversion efficiency ensures the initiation of hyperthermia and remains to be one of the most important parameters for the evaluation of photothermal agents. Secondly, the sufficient accumulation and distribution of photothermal agents at tumor sites not only guarantee the targeted therapy but also reduce the possible side effect, which makes them attractive factors that have attracted much attention. Thirdly, the combination of photothermal therapy with other therapeutic modalities (e.g., chemotherapy, photodynamic therapy, gene therapy) offers synergistic enhancement of anticancer effect and serves as an alternative strategy for the further optimization of photothermal therapy.
Host-guest assemblies, with the inclusion of small molecular guests into the cavity of macrocyclic hosts, are attractive to supramolecular chemists. They can be constructed via the manipulation of the size and non-covalent interactions (e.g., hydrogen bonding, electrostatic interaction, - interaction, hydrophobic interaction) between host and guest[15-18]. Due to their easy design, morphological diversity, stimuli-responsive property, etc., the host-guest assemblies have been broadly applied in the field of chemosensing[19,20], catalysis[21,22] and bio-applications[23-26]. Particularly, in the area of photothermal therapy, the host-guest assemblies show multiple advantages and significantly promote the improvement of cancer treatment. However, the related endeavors have not been systematically summarized and discussed. Thus, in this review, to benefit the design of advanced host-guest assembly-based photothermal agents and promote the further development of photothermal therapy, the major achievements that the host-guest assemblies have made in the field of photothermal therapy [Scheme 1], including the enhancement of photothermal conversion efficiency, the improvement of targeted distribution at tumor sites, and the superiority of constructing photothermal-derived multimodal synergistic therapy, are mainly discussed. Additionally, the future perspectives of host-guest assemblies-based photothermal therapy are envisioned. Overall, the review clarifies the unique function of host-guest assemblies in photothermal therapy and, more importantly, provides guidance for the development of host-guest assemblies-based cutting-edge photothermal therapeutic modality.
HOST-GUEST INTERACTION-ACTUATED ENHANCEMENT OF PHOTOTHERMAL CONVERSION EFFICIENCY
Though photothermal therapy has attracted much attention, the insufficient photothermal conversion efficiency in NIR range remains an obstacle to its clinical application[27,28]. Therefore, the optimization of the photothermal agents in this aspect is emerging as an appealing research direction. In addition, the NIR light employed for photothermal therapy is mostly limited to NIR-I (750-1,000 nm)[29-32]. However, compared with NIR-I, NIR-II (1,000-1,350 nm) is featured with larger penetration depth and maximum permissible exposure[33,34] according to American National Standard for Safe Use of Lasers. Hence, the construction of photothermal agents that function in NIR-II range is of significant importance for the development of photothermal therapy as well. Host-guest interaction provides a unique microenvironment[35,36], which may induce the redshift of the UV-Vis-NIR absorption of guest molecules. In addition, the occurrence of host-guest interaction for surface-modified nanomaterials can cause variation in their supramolecular morphology[37], which, to some degree, facilitates the manipulation of photothermal conversion efficiency both in NIR-I and NIR-II range. In this section, the enhancement of photothermal efficiency of photothermal agents promoted by host-guest interaction is exemplified and the underlying principles are clarified.
Host-guest interaction-induced enhancement of photothermal conversion efficiency in NIR-I
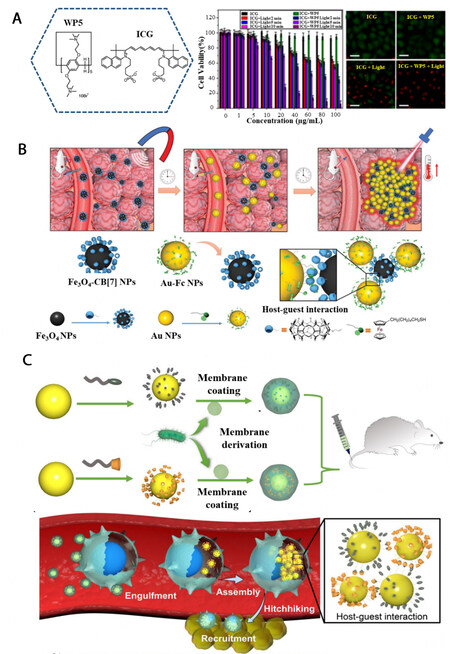
For host-guest assembly, the inner cavity of the macrocyclic host can provide a unique microenvironment for guest molecules, which, in some cases, can induce the enhancement of the photothermal conversion efficiency of the guest. ICG is a well-known NIR-I absorptive reagent. Because of its excellent biocompatibility, it is widely applied in photothermal therapy[38,39]. However, in aqueous solution, ICG is easily aggregated[40], which will decrease its photothermal conversion efficiency. Moreover, upon laser irradiation, ICG can undertake decomposition, which further attenuates its photothermal capability[41]. To overcome these problems, Ding et al. prepared quaternary ammonium cations-modified water-soluble pillar[5]arene (WP5) [Figure 1A][42]. Through hydrophobic and electrostatic interaction, ICG can be encapsulated into the cavity of WP5 and form host-guest complexes. In contrast with the individual ICG solution, the higher photothermal conversion efficiency was observed for the WP5 ⊃ ICG complex under 808 nm laser irradiation, indicating that ICG can be prevented from aggregation or degradation by forming host-guest assembly. The cellular experiments further confirmed the improved photothermal cancer therapeutic effect of WP5 ⊃ ICG.
Figure 1. The enhancement of photothermal conversion efficiency by host-guest interaction between (A) WP5 and ICG (reproduced with permission from ref.[42], Copyright 2021, Frontiers Media S.A); (B) Fe3O4-CB[7] and Au-Fc (reproduced with permission from ref.[46], Copyright 2021, John Wiley & Sons); (C) -CD-modified Au nanoparticles and Ada-modified Au nanoparticles (reproduced with permission from ref.[47], Copyright 2022, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science).
Au nanomaterials are common agents for photothermal therapy[43,44]. However, their low photothermal conversion efficiency in NIR range due to their small size hinders their application in photothermal therapy. While upon aggregation, the plasmonic coupling interaction between adjacent Au nanospecies can enhance their NIR absorption and photothermal conversion efficiency[45]. Inspired by the above fact, Cheng et al. prepared ferrocene (Fc)-capped Au nanoparticles and cucurbit[7]uril (CB[7])-capped Fe3O4 NPs, with average diameters of 14.04 nm and 18.53 nm, respectively [Figure 1B][46]. For individual Au-Fc or Fe3O4-CB[7], the photothermal conversion efficiency was limited to 9.1% or 8.7%. However, owing to the host-guest interaction between Fc and CB[7], intensive aggregation occurred with the simultaneous presence of Au-Fc and Fe3O4-CB[7] and the supramolecular aggregates exhibited significantly enhanced absorption around 808 nm. The temperature raising of Au-Fc/Fe3O4-CB[7] host-guest assemblies upon 808 nm laser irradiation was demonstrated to be as high as 35 °C and the corresponding photothermal conversion efficiency was increased to 36.7%, which is ~4 times higher than that of individual Au-Fc or Fe3O4-CB[7]. When applied in biological systems, the Fe3O4-CB[7] was accumulated in tumor tissue by an external magnetic field followed by the injection of Au-Fc. Contributed by the host-guest interaction, the Au-Fc displayed directional movement towards Fe3O4-CB[7], which eventually led to the formation of host-guest based aggregates and enhanced photothermal conversion efficiency at tumor sites. This concept was further strengthened by Gao et al.[47]. In this case, the -CD-modified Au nanoparticles and adamantane (Ada)-modified Au nanoparticles were first decorated with Escherichia coli outer membrane vesicles, respectively [Figure 1C]. With the bacterial-mimicking nature, the Au nanoparticles were easily phagocytosed by the immune cells in vivo. After the degradation of the Escherichia coli outer membrane vesicles by immune cells, the Au nanoparticles were released and the host-guest interaction between -CD and Ada led to their aggregation and enhanced photothermal conversion efficiency.
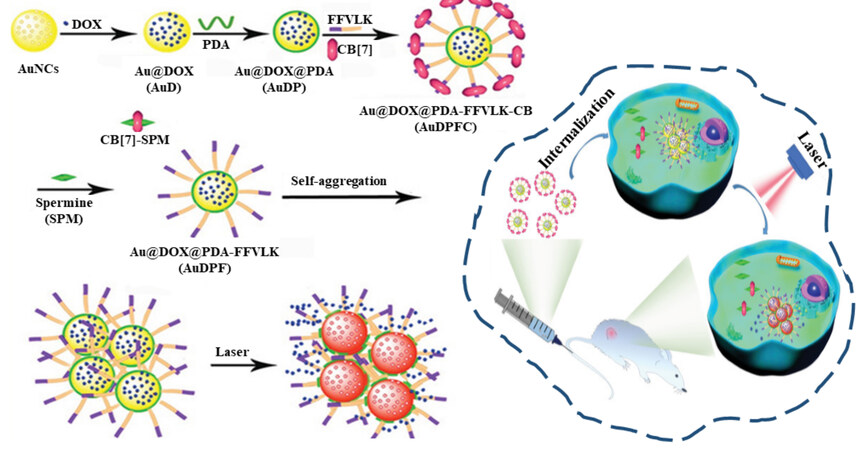
In other cases, the unique microenvironment of tumor site[48-50] can drive the reorganization of host-guest assembly, which will then affect the photothermal conversion efficiency of host-guest assembly-based agents. Spermine, which overexpresses in some types of tumors (e.g., breast cancer), was employed as external stimuli for the photothermal performance enhancement of host-guest assemblies. Wang et al. prepared polydopamine (PDA) coated nanocages (AuNCs), followed by the conjugation of a small peptide, Phe-Phe-Val-Leu-Lys (FFVLK), on the PDA shell via covalent bonds [Figure 2][51]. In the presence of CB[7], the terminal Phe unit of the FFVLK was incorporated into the cavity of CB[7] via host-guest interaction, resulting in the nanoassemblies of Au@PDA-FFVLK-CB. When accumulated in breast tumor tissue, due to the strong interaction between the over-expressed spermine and CB[7], the CB[7] was displaced from the Au@PDA-FFVLK, leaving the exposure of FFVLK. Owing to the intermolecular interaction of FFVLK via hydrogen bonds, hydrophobic interaction and π-π interaction, the self-aggregation of AuNCs occurred. As a result of the enhanced plasmonic coupling effect of AuNCs aggregates, the enhancement of photothermal conversion efficiency and photothermal therapeutic effect was achieved.
Figure 2. Overexpressed spermine at tumor sites induced enhancement of photothermal conversion efficiency of Au@PDA-FFVLK-CB host-guest assemblies. Reproduced with permission from ref.[51], Copyright 2022, John Wiley & Sons.
Host-guest interaction-induced enhancement of photothermal conversion efficiency in NIR-II
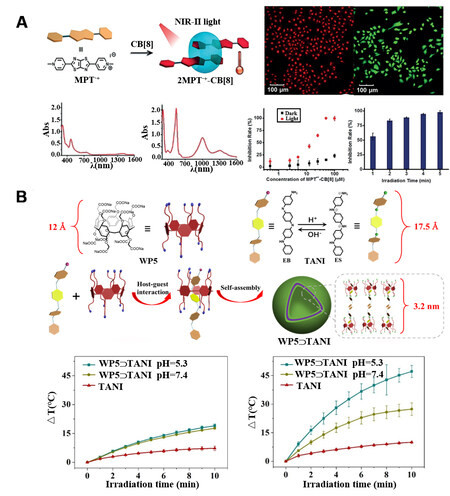
Constructing photothermal agents that function in NIR-II range is of great significance. The common strategy to achieve this goal lies in the extension of the -conjugation of specific compounds[52]. However, the above process requires elaborate design and excellent synthetic skills. To extend the NIR absorption of photothermal agents into NIR-II range in a more flexible manner, Tang et al. set an example by exploiting host-guest interaction[53]. They fabricated supramolecular radical dimer utilizing the host-guest interaction between N,N’-dimethylated dipyridinium thiazolo[5,4-d]thiazole (MPT2+) and cucurbit[8]uril (CB[8]) [Figure 3A]. It was demonstrated that, with the encapsulation inside CB[8], the two MPT2+ molecules were located in a parallel but staggered way. When reduced with photo-irradiation, radical dimers of 2MPT·+-CB[8] with the dramatical enhancement of NIR-II absorption at 1004 nm were formed due to the photoinduced electron transfer. However, compared to the individual MPT2+, only a small number of radical dimers were formed, and in this case, weak absorption within NIR-II range was observed, confirming the radical stabilizing function of the macrocyclic hosts. In addition, the photothermal conversion efficiency of 2MPT·+-CB[8] was demonstrated to be 54.6% with the 1064 nm laser irradiation. Moreover, the effective inhibition of HepG2 cancer cells was confirmed with 2MPT·+-CB[8] as photothermal agent.
Our group further extended the above principle by using WP5 as host and aniline tetramer (TANI) as guest [Figure 3B][54]. For TANI itself, the maximum UV-Vis absorption is around 550 nm, with negligible signal in the NIR range. However, when interacting with WP5, supramolecular vesicles actuated by the host-guest interaction were formed, and much higher absorption at 1064 nm was observed. The host-guest assembly-promoted significant change in NIR absorption was ascribed to the enhanced π-π interaction during the assembly process, which benefited the conjugation extension. The WP5⊃TANI supramolecular assemblies with enhanced NIR-II absorption and photothermal performance showed outstanding photothermal therapeutic efficacy as demonstrated by both in vitro and in vivo results.
In summary, the construction of host-guest assemblies has been proven as a promising strategy to improve photothermal conversion efficiency of specific organic molecules or even nanomaterials. For organic molecules, on the one hand, the formation of host-guest assemblies can offer a unique microenvironment, which acts as a shelter for NIR absorptive agents and improves their photothermal stability and, further, photothermal capability. On the other hand, the occurrence of host-guest interaction and host-guest complex actuated assembly can induce the extension of -conjugation in a supramolecular way, which leads to the redshift of absorption spectrum and, therefore, enhanced photothermal conversion efficiency in NIR biowindow. For nanomaterials, their photothermal conversion efficiency is closely related to size or supramolecular arrangement. Therefore, through the host-guest interaction-induced size or supramolecular arrangement, their photothermal performance can be flexibly manipulated.
HOST-GUEST INTERACTION-ACTUATED DEEP TUMOR PENETRATION OF PHOTOTHERMAL AGENTS
The efficient distribution of photothermal agents to tumor issues is important for photothermal therapy. In many reported cases, the photothermal agents accumulated at tumor sites via the enhanced permeability and retention effect[55,56]. It has been demonstrated that nanomaterials with relatively large sizes, 200 nm, have higher efficiency of accumulation at tumor sites[57,58]. However, due to the high interstitial fluid pressure and dense extracellular matrix, it is difficult for large nanospecies to penetrate deeply into tumor tissues[59]. Given the contradictory size requirements between tumor accumulation and penetration, tumor environment stimulated size switch of photothermal agents, undoubtedly, will be a reasonable solution to facilitate both cases and, further, the performance of photothermal therapy.
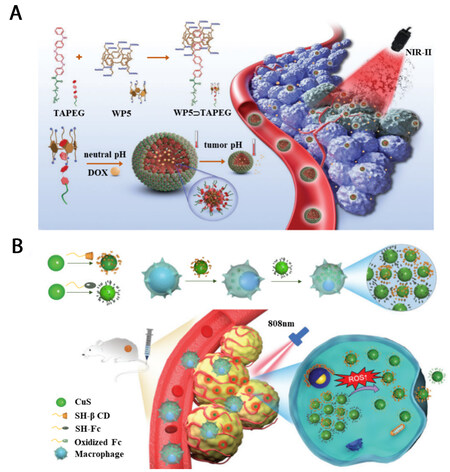
Our group reported host-guest assemblies with polyethylene glycol-modified aniline tetramer (TAPEG) and WP5 [Figure 4A][60]. At pH 7.4, it was demonstrated that the WP5⊃TAPEG formed core-shell architecture with an average size of 200 nm through multi-micelle aggregation mechanism. However, at pH 4.5, due to the proton doping of TAPEG, the hydrophobicity of the WP5⊃TAPEG was decreased and the intermicelle aggregation was interrupted. As a result of the acidic condition, micelles with an average diameter of 20 nm were observed. The tumor pH-induced size switch behavior of WP5⊃TAPEG-based host-guest assemblies perfectly promoted the high accumulation and deep penetration at tumor sites, which further resulted in improved photothermal tumor therapy.
Figure 4. (A) Tumor acidic environment induced size switch and deep penetration of WP5⊃TAPEG host-guest assemblies. Reproduced with permission from ref.[60], Copyright 2022, John Wiley & Sons; (B) Overexpressed hydrogen peroxide-induced size switch and deep penetration of CD-CuS and Fc-CuS based host-guest assemblies. Reproduced with permission from ref.[61], Copyright 2021, the Royal Society of Chemistry.
Wang et al. reported another size switch related photothermal therapy utilizing host-guest interaction
From the abovementioned examples, it is obvious that a tumor microenvironment-triggered size switch system is advantageous for photothermal cancer therapy. To design photothermal agents with size switchable features, the on/off interaction, i.e., stimuli-responsive interaction, among small-sized photothermal species is an important factor that needs to be considered. Given that host-guest assemblies are featured with stimuli-responsive performance, they are regarded as excellent candidates for the construction of size switch photothermal systems under tumor microenvironment (e.g., low pH, concentrated GSH, high amount of H2O2).
HOST-GUEST INTERACTION-ACTUATED PHOTOTHERMAL THERAPY-DERIVED SYNERGISTIC THERAPY
Synergistic therapy is emerging as a popular direction for the improvement of cancer therapy[62,63]. To further optimize the photothermal curative efficacy, the construction of photothermal-based synergistic therapy (i.e., chemo-photothermal therapy, photodynamic-photothermal therapy, immunotherapy-photothermal therapy) is considered as an alternative strategy. This section discusses the superiority of host-guest assemblies from the perspective of synergistic therapy.
Chemo-photothermal therapy
Host-guest assemblies are outstanding candidates for smart drug nanocarriers owing to their versatile morphology and stimuli-responsive behavior. There are three common methodologies for the engineering of host-guest-based drug delivery systems: (1) The drug molecule is covalently linked with either host or guest component and then involved in the host-guest assembly process[64]; (2) the drug molecule itself acts as a guest and then participates in the host-guest assembly process[65]; and (3) the host-guest assemblies are prepared in advance, followed by the encapsulation of drug molecules[66]. In addition, besides the flexibility in drug encapsulation, host-guest interaction controls stimuli-responsive behavior-induced targeted drug release, which is another important factor for drug delivery systems.
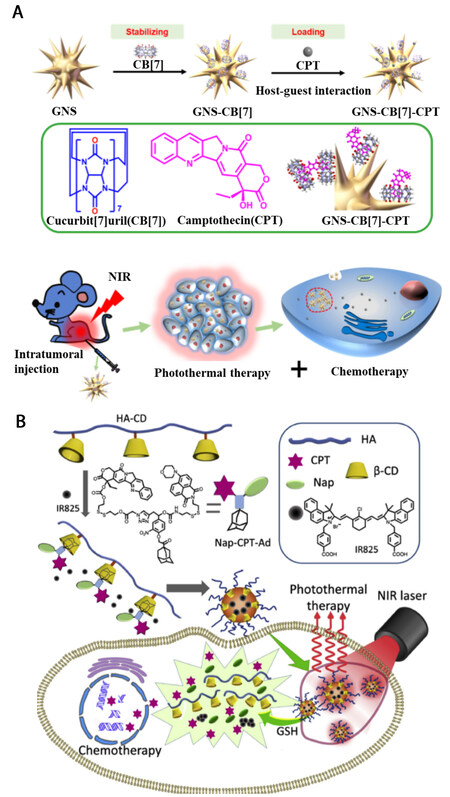
Given that the host-guest assemblies are not only beneficial for the construction of photothermal agents but also demonstrated as ideal platforms for drug delivery, the combination of the above dual functions makes them excellent candidates for chemo-photothermal synergistic therapy. Xu et al. prepared CB[7] stabilized AuNRs [Figure 5A][67]. Taking advantage of the hydrophobic cavity of CB[7], the anticancer drug, camptothecin (CPT), was encapsulated through host-guest interaction. As the AuNRs showed intensive absorption around 800 nm, they can induce a temperature increase of 40 °C with 808 nm laser irradiation, which fully meets the requirement of photothermal therapy. Moreover, upon laser irradiation, the CPT can be controllably released due to the high temperature actuated disassociation of the host-guest complexation. According to the in vivo experiments, the synergistic chemo-photothermal therapy induced effective inhibition of tumor growth.
Figure 5. Schematic illustration of chemo-photothermal synergistic therapy utilizing the host-guest interaction between (A) CB[7] and CPT (reproduced with permission from ref.[67], Copyright 2018, American Chemical Society); (B) CD and CPT (reproduced with permission from ref.[68], Copyright 2022, Elsevier B.V.)
Besides the high temperature triggered drug release, tumor microenvironment-induced controllable drug release was designed for chemo-photothermal therapy as well. Zhao et al. synthesized CD-modified HA[68]. In the presence of CPT-modified Ada derivative, the host-guest interaction between CD and Ada promoted the formation of supramolecular nanoparticles [Figure 5B]. With the loading of NIR absorptive dye, IR825, the host-guest assemblies can cause a temperature increase of 40 °C upon laser irradiation, confirming their excellent photothermal performance. In addition, the anticancer drug, CPT, was deliberately linked with Ada by a disulfide bond, which can be cleaved by the concentrated GSH in tumor microenvironment. Evidence showed that the combination of chemo and photothermal therapy ensured the efficient inhibition of tumor growth.
Photodynamic-photothermal therapy
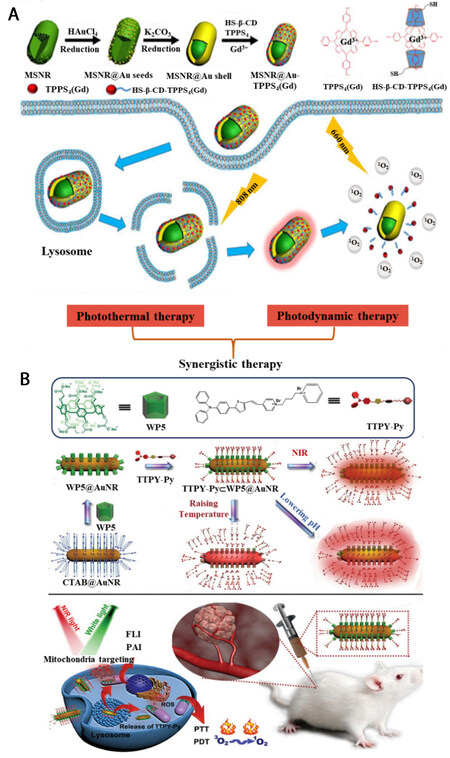
Photodynamic therapy is an emerging tumor treatment that uses light along with photosensitizer, which can transfer laser energy to the surrounding oxygen, and produce reactive oxygen species (e.g., 1O2)[9,69]. The combination of both photothermal therapy and photodynamic therapy will undoubtedly improve cancer curative effects. One of the common strategies for constructing photodynamic-photothermal agents is to encapsulate photosensitizers into photothermal agents utilizing host-guest interaction. Yang et al. fabricated mesoporous silica-coated Au nanorods through a seed-induced growth method[70], which exhibited relatively high photothermal conversion efficiency and can be employed for photothermal therapy [Figure 6A]. Taking advantage of the Au surface, HS-modified -CD was covalently linked. Via host-guest interaction, 5,10,15,20-Tetrakis (4-sulfonatophenyl)-porphyrin (TPPS4) coordinated with Gd3+, which acted as a photosensitizer, was encapsulated into the nanocomposite, resulting the obtainment of MSNR@Au-TPPS4(Gd). With 808 nm laser irradiation, the MSNR@Au-TPPS4(Gd) showed excellent photothermal behavior. While upon the irradiation of 660 nm laser, 1O2 was generated as well. With the combination of the dual methodologies, the improved therapeutic effect was confirmed by in vivo experiments. This principle was further expanded by Tang et al. [Figure 6B][71]. In their case, TTPY-Py, an AIE-active photosensitizer, was incorporated into WP5-modified Au nanorods via host-guest interaction. Taking TTPY-Py as a photosensitizer for the generation of reactive oxygen species and Au nanorods as photothermal agents, the combination of photodynamic and photothermal synergistic therapy was realized and an enhanced anti-tumor effect was achieved.
Figure 6. Schematic illustration of photodynamic-photothermal synergistic therapy taking advantage of the host-guest interaction between (A) CD and TPPS4(Gd) (reproduced with permission from ref.[70], Copyright 2019, American Chemical Society); (B) WP5 and TTPY-Py (reproduced with permission from ref.[71], Copyright 2022, John Wiley & Sons).
Other photothermal-based synergistic therapies
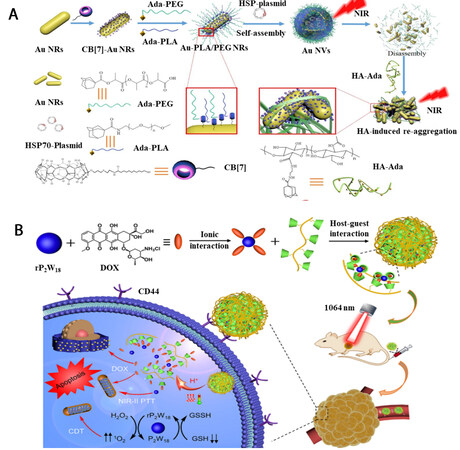
The incorporation of therapeutic genes with photothermal species leads to the construction of gene-photothermal agents. Yue et al. prepared CB[7]-capped AuNRs, Ada-PEG and Ada-PLA (polylactic acid) [Figure 7A]. When mixed together, the host-guest interaction between CB[7] and Ada occurred. Further assisted with the hydrophobic (PLA) and hydrophilic (PEG) ligands, supramolecular vesicles (Au NVs) with densely arranged AuNRs were obtained[72]. The Au NVs were demonstrated with enhanced photothermal behavior than that of individual AuNRs, owing to the plasmonic coupling effect of AuNRs inside the NVs. In addition, the Au NVs can incorporate plasmid. Upon mild laser irradiation, the obtained Au NVs underwent disassociation, initiating the release of plasmid followed by the spatiotemporal gene expression at tumor sites. Intriguingly, with the later delivery of Ada-modified polysaccharide HA into cancer cells, the reactivation of host-guest interaction promoted the aggregation of AuNRs, offering the possibility of gene-photothermal synergistic therapy.
Figure 7. Schematic illustration of (A) gene-photothermal synergistic therapy taking advantage of the host-guest interaction between CB[7] and Ada (reproduced with permission from ref.[72], Copyright 2021, Chinese Chemical Society; This Figure has been published in CCS Chemistry [2021]; [Supramolecular Vesicles Based on Gold Nanorods for Precise Control of Gene Therapy and Deferred Photothermal Therapy] is available online at [10.31635/ccschem.021.202101029; https://www.chinesechemsoc.org/doi/10.31635/ccschem.021.202101029]; (B) chemo-chemodynamic-photothermal synergistic therapy based on rP2W18 and host-guest interaction between CD and DOX (reproduced with permission from ref.[73], Copyright 2022, American Chemical Society).
Three-in-one cancer therapy, via the combination of photothermal therapy, chemodynamic therapy and chemotherapy, was constructed by Kong et al. utilizing host-guest interaction [Figure 7B][73]. In this case, a reduced polyanionic cluster (rP2W18) was employed as the core structure for the encapsulation of DOX, followed by the grafting of β-CD modified hyaluronic acid through host-guest interaction between DOX and β-CD. The as-prepared rP2W18-based nanomaterials showed excellent photothermal behavior under laser irradiation. In addition, upon tumor acidic microenvironment and laser irradiation, the host-guest interaction was disrupted, followed by the release of DOX and initiation of chemotherapy. Moreover, the rP2W18 cluster was featured with the capability to reduce overexpressed oxidants at tumor sites, further offering chemodynamic therapy.
CONCLUSION AND OUTLOOK
In summary, proved by the recent progress, it is safe to conclude that the host-guest interaction can be taken as an efficient weapon for the improvement of photothermal cancer therapy. Through host-guest interaction, the biocompatibility, stability and photothermal conversion efficiency of photothermal agents can be optimized. In addition, upon the tumor microenvironment, the size switch of the host-guest assemblies can be achieved owing to their stimuli-responsive performance, which facilitates the accumulation and deep penetration of photothermal agents to tumor sites.
Moreover, the host-guest assemblies provide additional superiorities for the construction of photothermal therapy-based synergistic therapy. Though milestones have been achieved in the field of host-guest assembly-based photothermal therapy, efforts still need to be made to promote its widespread clinical application. Firstly, temperature imaging can visually provide information for photothermal cancer therapy. Therefore, the combination of temperature-responsive imaging species and photothermal agents through host-guest interaction could make an appealing direction for future research. Secondly, the precise control of the temperature rise of photothermal agents can guarantee both satisfying therapeutic efficacy and negligible side effect. Hence, the development of photothermal therapy with controllable hyperthermia, through the manipulation of host-guest interaction-induced change of photothermal conversion efficiency, should be extensively investigated. Last but not least, chiral host-guest assemblies can be employed to regulate the interaction between photothermal agents and cancer cells, which further provides an alternative strategy for the improvement of photothermal cancer therapy.
DECLARATIONS
Authors’ contributionsConceptualization: Han J, Sun X, Guo R
Writing-original draft preparation: Sun X
Writing-review and editing: Ye Q, Zhou J, Han J
Read and agreed to the published version of the manuscript: Sun X, Ye Q, Zhou J, Han J, Guo R
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China (21922202, 22002138 and 22272146); Chinese Postdoctoral Science Foundation (2021M692714); the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Du X, Li Y, Long J, et al. Fabrication of cisplatin-loaded polydopamine nanoparticles via supramolecular self-assembly for photoacoustic imaging guided chemo-photothermal cancer therapy. Appl Mater 2021;23:101019.
2. Song IA, Park HY, Oh TK. Effect of preoperative psychiatric morbidity on postoperative outcomes of lung cancer surgery: a nationwide cohort study in South Korea. J Psychosom Res 2022;161:111002.
3. Hong S, Li Z, Li C, Dong C, Shuang S. β-Cyclodextrin grafted polypyrrole magnetic nanocomposites toward the targeted delivery and controlled release of doxorubicin. App Surf Sci 2018;427:1189-98.
4. Zhao Q, Li X, Lu J, et al. TPGS and cypate gated mesoporous carbon for enhanced thermochemotherapy of tumor. Colloids Surf A Physicochem Eng Asp 2020;591:124544.
5. Sun R, Ge Y, Liu H, He P, Song W, Zhang X. Erythrocyte membrane-encapsulated glucose oxidase and manganese/ferrite nanocomposite as a biomimetic “all in one” nanoplatform for cancer therapy. ACS Appl Bio Mater 2021;4:701-10.
6. Yin H, Zhou B, Zhao C, et al. 2D core/shell-structured mesoporous silicene@silica for targeted and synergistic NIR-II-Induced photothermal ablation and hypoxia-activated chemotherapy of tumors. Adv Funct Mater 2021;31:2102043.
7. Niu Q, Sun Q, Bai R, et al. Progress of nanomaterials-based photothermal therapy for oral squamous cell carcinoma. Int J Mol Sci 2022;23:10428.
8. Chen H, Zeng X, Tham HP, et al. NIR-light-activated combination therapy with a precise ratio of photosensitizer and prodrug using a host-guest strategy. Angew Chem Int Ed Engl 2019;58:7641-6.
9. Chen H, Sun T, Zeng W, et al. NIR-light-intensified hypoxic microenvironment for cascaded supra-prodrug activation and synergistic chemo/photodynamic cancer therapy. ACS Materials Lett 2022;4:111-9.
10. Li QL, Wang D, Cui Y, et al. AIEgen-functionalized mesoporous silica gated by cyclodextrin-modified cus for cell imaging and chemo-photothermal cancer therapy. ACS Appl Mater Interfaces 2018;10:12155-63.
11. Wu MX, Yan HJ, Gao J, et al. Multifunctional supramolecular materials constructed from polypyrrole@uio-66 nanohybrids and pillararene nanovalves for targeted chemophotothermal therapy. ACS Appl Mater Interfaces 2018;10:34655-63.
12. Xie Y, Wang M, Sun Q, Wang D, Luo S, Li C. PtBi-β-CD-Ce6 nanozyme for combined trimodal imaging-guided photodynamic therapy and NIR-II responsive photothermal therapy. Inorg Chem 2022;61:6852-60.
13. Wei C, Jin X, Yin P, Wu C, Zhang W. Carbon spheres for photothermal therapy of tumor cells: rapid preparation and high photothermal effect. J Inorg Mater 2021;36:1208.
14. Yu Y, Tang D, Liu C, et al. Biodegradable polymer with effective near-infrared-ii absorption as a photothermal agent for deep tumor therapy. Adv Mater 2022;34:e2105976.
15. Herrera-españa AD, Höpfl H, Morales-rojas H. Host-guest properties of a trigonal iminoboronate ester cage self-assembled from hexahydroxytriphenylene. Eur J Org Chem 2022:2022.
16. Song Q, Yang J, Rho JY, Perrier S. Supramolecular switching of the self-assembly of cyclic peptide-polymer conjugates via host-guest chemistry. Chem Commun (Camb) 2019;55:5291-4.
17. Xie X, Gao B, Ma Z, et al. Host-guest interaction driven peptide assembly into photoresponsive two-dimensional nanosheets with switchable antibacterial activity. CCS Chem 2021;3:1949-62.
18. Xu D, Zhou Q, Dai X, et al. Cucurbit[8]uril-mediated phosphorescent supramolecular foldamer for antibiotics sensing in water and cells. Chin Chem Lett 2022;33:851-4.
19. Li Y, Wen J, Li J, Wu Z, Li W, Yang K. Recent applications of pillar[n]arene-based host-guest recognition in chemosensing and imaging. ACS Sens 2021;6:3882-97.
20. Xia D, Ma J, Wang P. A hydrogen sulfide-sensitive supramolecular polymer constructed by crown ether-based host-guest interaction and Ag-coordination. Sens Actuators B Chem 2019;279:197-203.
21. Teyssandier J, Feyter S, Mali KS. Host-guest chemistry in two-dimensional supramolecular networks. Chem Commun (Camb) 2016;52:11465-87.
22. Wang Z, Zhu H, Tu W, et al. Host/guest nanostructured photoanodes integrated with targeted enhancement strategies for photoelectrochemical water splitting. Adv Sci (Weinh) 2022;9:e2103744.
23. Zhang Y, Ma S, Liu X, et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv Mater 2021;33:e2007293.
24. Yu R, Yang Y, He J, Li M, Guo B. Novel supramolecular self-healing silk fibroin-based hydrogel via host-guest interaction as wound dressing to enhance wound healing. Chem Eng J 2021;417:128278.
25. Yang X, Wu B, Zhou J, et al. Controlling intracellular enzymatic self-assembly of peptide by host-guest complexation for programming cancer cell death. Nano Lett 2022;22:7588-96.
26. Yue L, Yang K, Lou X, Yang Y, Wang R. Versatile roles of macrocycles in organic-inorganic hybrid materials for biomedical applications. Matter 2020;3:1557-88.
27. Xu Z, Zhang Y, Zhou W, et al. NIR-II-activated biocompatible hollow nanocarbons for cancer photothermal therapy. J Nanobiotechnology 2021;19:137.
28. Song X, Lu X, Sun B, et al. Conjugated Polymer nanoparticles with absorption beyond 1000 nm for nir-ii fluorescence imaging system guided NIR-II photothermal therapy. ACS Appl Polym Mater 2020;2:4171-9.
29. Sun X, Wang J, Wang Z, et al. Gold nanorod@void@polypyrrole yolk@shell nanostructures: synchronous regulation of photothermal and drug delivery performance for synergistic cancer therapy. J Colloid Interface Sci 2022;610:89-97.
30. Wang J, Zhu C, Han J, et al. Controllable synthesis of gold nanorod/conducting polymer core/shell hybrids toward in vitro and
31. Wang J, Han J, Zhu C, et al. Gold nanorods/polypyrrole/m-SiO(2) core/shell hybrids as drug nanocarriers for efficient chemo-photothermal therapy. Langmuir 2018;34:14661-9.
32. Wang H, Xue KF, Yang Y, Hu H, Xu JF, Zhang X. In situ hypoxia-induced supramolecular perylene diimide radical anions in tumors for photothermal therapy with improved specificity. J Am Chem Soc 2022;144:2360-7.
33. Cen M, Ding Y, Wang J, et al. Cationic water-soluble pillar[5]arene-modified cu(2-x)se nanoparticles: supramolecular trap for atp and application in targeted photothermal therapy in the NIR-II window. ACS Macro Lett 2020;9:1558-62.
34. Yu Z, Chan WK, Zhang Y, Tan TTY. Near-infrared-II activated inorganic photothermal nanomedicines. Biomaterials 2021;269:120459.
35. Chu N, Cong L, Yue J, Xu W, Xu S. Fluorescent imaging probe targeting mitochondria based on supramolecular host-guest assembly and disassembly. ACS Omega 2022;7:34268-77.
36. Li H, Wei R, Yan GH, et al. Smart self-assembled nanosystem based on water-soluble pillararene and rare-earth-doped upconversion nanoparticles for ph-responsive drug delivery. ACS Appl Mater Interfaces 2018;10:4910-20.
37. Huang C, Zhang H, Hu Z, Zhang Y, Ji X. Enhancing mechanical performance of a polymer material by incorporating pillar[5]arene-based host-guest interactions. Gels 2022;8:475.
38. Yoon HJ, Lee HS, Lim JY, Park JH. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl Mater Interfaces 2017;9:5683-91.
39. Zhu S, Wang S, Liu C, Lyu M, Huang Q. Cu-hemin nanosheets and indocyanine green co-loaded hydrogel for photothermal therapy and amplified photodynamic therapy. Front Oncol 2022;12:918416.
40. Sun R, Liu M, Xu Z, Song B, He Y, Wang H. Silicon-based nanoprobes cross the blood—brain barrier for photothermal therapy of glioblastoma. Nano Res 2022;15:7392-401.
41. Lin L, Liang X, Xu Y, Yang Y, Li X, Dai Z. Doxorubicin and indocyanine green loaded hybrid bicelles for fluorescence imaging guided synergetic chemo/photothermal therapy. Bioconjug Chem 2017;28:2410-9.
42. Ding Y, Wang C, Lu B, Yao Y. Enhancing the stability and photothermal conversion efficiency of ICG by pillar[5]arene-based host-guest interaction. Front Chem 2021;9:775436.
43. Leng C, Zhang X, Xu F, et al. Engineering gold nanorod-copper sulfide heterostructures with enhanced photothermal conversion efficiency and photostability. Small 2018;14:e1703077.
44. Tang M, Zhang Z, Ding C, et al. Two birds with one stone: innovative ceria-loaded gold@platinum nanospheres for photothermal-catalytic therapy of tumors. J Colloid Interface Sci 2022;627:299-307.
45. Lin X, Ye S, Kong C, et al. Polymeric ligand-mediated regioselective bonding of plasmonic nanoplates and nanospheres. J Am Chem Soc 2020;142:17282-6.
46. Cheng Q, Yue L, Li J, et al. Supramolecular tropism driven aggregation of nanoparticles in situ for tumor-specific bioimaging and photothermal therapy. Small 2021;17:e2101332.
47. Gao C, Wang Q, Li J, et al.
48. Han R, Wu S, Yan Y, Chen W, Tang K. Construction of ferrocene modified and indocyanine green loaded multifunctional mesoporous silica nanoparticle for simultaneous chemodynamic/photothermal/photodynamic therapy. Mater Today Commun 2021;26:101842.
49. Hu XY, Gao L, Mosel S, et al. From supramolecular vesicles to micelles: controllable construction of tumor-targeting nanocarriers based on host-guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small 2018;14:e1803952.
50. Wang Y, Jin M, Chen Z, et al. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar[5]arene dimer for targeted chemotherapy. Chem Commun (Camb) 2020;56:10642-5.
51. Xie B, Zhao H, Shui M, et al. Spermine-responsive intracellular self-aggregation of gold nanocages for enhanced chemotherapy and photothermal therapy of breast cancer. Small 2022;18:e2201971.
52. Cui FH, Li Q, Gao LH, et al. Condensed osmaquinolines with NIR-II absorption synthesized by Aryl C-H annulation and aromatization. Angew Chem Int Ed Engl 2022;61:e202211734.
53. Tang B, Li WL, Chang Y, et al. A supramolecular radical dimer: high-efficiency NIR-II photothermal conversion and therapy. Angew Chem Int Ed Engl 2019;58:15526-31.
54. Chen X, Wang Z, Sun X, et al. Photothermal supramolecular vesicles coassembled from pillar[5]arene and aniline tetramer for tumor eradication in NIR-I and NIR-II biowindows. Chem Eng J 2021;403:126423.
55. Tang Z, Tian W, Long H, et al. Subcellular-targeted near-infrared-responsive nanomedicine with synergistic chemo-photothermal therapy against multidrug resistant cancer. Mol Pharm 2022;19:4538-51.
56. Zhong Z, Liu C, Xu Y, et al. γ-Fe(2) O(3) loading mitoxantrone and glucose oxidase for pH-responsive chemo/chemodynamic/photothermal synergistic cancer therapy. Adv Healthc Mater 2022;11:e2102632.
57. Bai S, Zhang Y, Li D, Shi X, Lin G, Liu G. Gain an advantage from both sides: Smart size-shrinkable drug delivery nanosystems for high accumulation and deep penetration. Nano Today 2021;36:101038.
58. Gu Z, Dong Y, Xu S, Wang L, Liu Z. Molecularly imprinted polymer-based smart prodrug delivery system for specific targeting, prolonged retention, and tumor microenvironment-triggered release. Angew Chem Int Ed Engl 2021;60:2663-7.
59. Li HJ, Du JZ, Liu J, et al. Smart superstructures with ultrahigh ph-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano 2016;10:6753-61.
60. Wang Z, Wang Y, Sun X, et al. Supramolecular core-shell nanoassemblies with tumor microenvironment-triggered size and structure switch for improved photothermal therapy. Small 2022;18:e2200588.
61. Li J, Cheng Q, Yue L, et al. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz 2021;6:907-12.
62. Ren Z, Sun S, Sun R, et al. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv Mater 2020;32:e1906024.
63. Sun P, Deng Q, Kang L, Sun Y, Ren J, Qu X. A smart nanoparticle-laden and remote-controlled self-destructive macrophage for enhanced chemo/chemodynamic synergistic therapy. ACS Nano 2020;14:13894-904.
64. Ding Y, Wang C, Ma Y, et al. pH/ROS dual-responsive supramolecular polypeptide prodrug nanomedicine based on host-guest recognition for cancer therapy. Acta Biomater 2022;143:381-91.
65. Xue F, Wang Y, Zhang Q, et al. Self-assembly of affinity-controlled nanoparticles via host-guest interactions for drug delivery. Nanoscale 2018;10:12364-77.
66. Sun G, Zuo M, Xu Z, Wang K, Wang L, Hu XY. Orthogonal design of supramolecular prodrug vesicles via water-soluble pillar[5]arene and betulinic acid derivative for dual chemotherapy. ACS Appl Bio Mater 2022;5:3320-8.
67. Xu P, Feng Q, Yang X, et al. Near infrared light triggered cucurbit[7]uril-stabilized gold nanostars as a supramolecular nanoplatform for combination treatment of cancer. Bioconjug Chem 2018;29:2855-66.
68. Zhang Y, Yang D, Chen H, et al. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018;163:14-24.
69. Wang H, Han RL, Yang LM, et al. Design and synthesis of core-shell-shell upconversion nanoparticles for NIR-induced drug release, photodynamic therapy, and cell imaging. ACS Appl Mater Interfaces 2016;8:4416-23.
70. Yang S, You Q, Yang L, et al. Rodlike MSN@Au nanohybrid-modified supermolecular photosensitizer for NIRF/MSOT/CT/MR quadmodal imaging-guided photothermal/photodynamic cancer therapy. ACS Appl Mater Interfaces 2019;11:6777-88.
71. Song N, Zhang Z, Liu P, et al. Pillar[5]arene-modified gold nanorods as nanocarriers for multi-modal imaging-guided synergistic photodynamic-photothermal therapy. Adv Funct Mater 2021;31:2009924.
72. Yue L, Yang K, Wei J, et al. Supramolecular vesicles based on gold nanorods for precise control of gene therapy and deferred photothermal therapy. CCS Chem 2022;4:1745-57.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Sun X, Ye Q, Zhou J, Han J, Guo R. Host-guest assemblies for improved photothermal cancer therapy. Chem Synth 2023;3:16. http://dx.doi.org/10.20517/cs.2022.45
AMA Style
Sun X, Ye Q, Zhou J, Han J, Guo R. Host-guest assemblies for improved photothermal cancer therapy. Chemical Synthesis. 2023; 3(2): 16. http://dx.doi.org/10.20517/cs.2022.45
Chicago/Turabian Style
Sun, Xiaohuan, Qianyun Ye, Jinfeng Zhou, Jie Han, Rong Guo. 2023. "Host-guest assemblies for improved photothermal cancer therapy" Chemical Synthesis. 3, no.2: 16. http://dx.doi.org/10.20517/cs.2022.45
ACS Style
Sun, X.; Ye Q.; Zhou J.; Han J.; Guo R. Host-guest assemblies for improved photothermal cancer therapy. Chem. Synth. 2023, 3, 16. http://dx.doi.org/10.20517/cs.2022.45
About This Article
Special Issue
Copyright
Author Biographies





Data & Comments
Data
 Cite This Article 27 clicks
Cite This Article 27 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.