Application of sulfonium and sulfoxonium ylides in organocatalyzed asymmetric reaction
Abstract
Sulfur-based ylides are very important and valuable reagents in organic synthesis and have been widely applied in the preparation of cyclic compounds and the formation of C-X (X = C, N, O, S, B, P) bond. Since the early 2000s, asymmetric organocatalysis has become a powerful strategy in organic synthesis (even highlighted by the 2021 Nobel Prize in Chemistry) and has attracted widespread research interest among synthetic organic chemists. In this area, a large number of outstanding achievements have been registered in the novel chiral catalysts design, new compounds synthesis, various reagents application, and versatile reaction discovery. Given the extensive application of sulfonium and sulfoxonium ylides in organic synthesis, in this review, we mainly summarize the organocatalyzed asymmetric reactions involving these ylide reagents as one of the reactants. Detailed reaction mechanisms, transition states and some synthetic applications are highlighted. The challenges and opportunities of this field are also discussed in the outlook.
Keywords
INTRODUCTION
Sulfur-containing compounds are very important in organic chemistry, not only because of their prevalence in a number of fascinating natural products, clinical drugs, and pharmaceutical candidates[1-3], but also due to their applicability as chiral auxiliary, chiral ligands, or chiral organocatalysts for various asymmetric transformations[4-6]. Actually, a large number of organosulfur reagents have been identified and confirmed as versatile reactants for many different kinds of reactions[7,8]. However, among the diverse prominent organosulfur reagents, sulfur-based ylides should belong to the most important and widely-used sulfur-containing reactants in the realm of organic synthetic chemistry. Through literature survey and search, it was found that since the pioneering work of Ingold and Jessop[9] and the groundbreaking work of Johnson and Lacount[10], Corey and Chaykovsky in the 1960s[11-14], the study on sulfur-based ylides chemistry has achieved an impressive development during the past nearly 60 years[15-19]. In this area, especially, stabilized sulfonium and sulfoxonium ylides bearing a carbonyl group at the α-carbon atom [Figure 1] have occupied a dominant position, probably due to some of their inherent characteristics including thermal stability, easy preparation and use, and low toxicity, as well as owing to their powerful functions as nucleophilic 1,1’-dipolar species for realizing numerous chemical transformations. Moreover, with the rapid development of catalytic asymmetric synthesis, several stereocontrolled strategies, including asymmetric organometallic catalysis and organocatalysis, have been implemented in the reactions involving sulfonium and sulfoxonium ylides for the construction of chiral compounds.
With the increase of reports on various reactions involving sulfonium and sulfoxonium ylides, several reviews concerning the application of sulfonium and sulfoxonium ylides in organic synthetic chemistry have been published in recent years. In 2017, Lu et al. summarized the catalytic cyclization reactions of sulfur ylides[20]. In 2018, Neuhaus et al. reported the developments in the transition metal-catalyzed reactions involving sulfonium and sulfoxonium ylides[21]. One year later, a review on the bond-forming and -breaking reactions from organic sulfur(IV) molecules including sulfonium salts and sulfur ylides was also published from the same group[22]. Subsequently, the versatile applications of sulfoxonium ylides in organic synthesis were also reviewed by Bernardi’s and Burtoloso’s groups, respectively[23,24]. In addition, Fan et al. also published an overview of sulfonium salt and sulfur ylide chemistry[25]. Although these reviews have been published, a critical survey of the literature reveals that there are a lot of elegant works on the catalytic asymmetric reactions of sulfonium and sulfoxonium ylides by organocatalysis has been reported, but no special review summarizes these works. In this context, based on a serious consideration involving the rapid development of asymmetric organocatalysis and the wide application of sulfur-based ylides in organic synthesis, a comprehensive review on this aspect is needed.
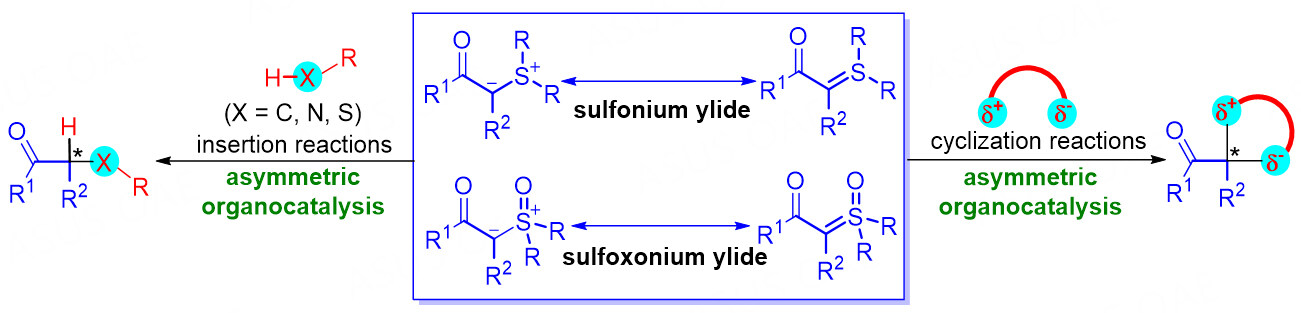
It is an indisputable fact that asymmetric organocatalysis has emerged as a powerful strategy in organic synthesis for the construction of chiral molecule scaffolds since the early 2000s[26-29]. In this research area, a large number of outstanding achievements have been made from various aspects. Many useful reagents have been successfully applied to diverse organocatalyzed asymmetric reactions, and the sulfonium and sulfoxonium ylides are not an exception. In this review, we will comprehensively present the application of sulfonium ylides and sulfoxonium ylides in asymmetric chemical transformations with organocatalysis tactics. In addition, the substrate scope, possible mechanism, transition states, and synthetic applications are also introduced. For better understanding, this review is classified into two main sections according to the type of sulfur-based ylides: (1) organocatalytic asymmetric reactions of sulfonium ylides; (2) organocatalytic asymmetric reactions of sulfoxonium ylides. In each section, the reaction types include both asymmetric X-H (X = C, N, S) bond insertion reactions and asymmetric cyclization reactions [Figure 2]. In addition, it should be declared here that this review only focuses on the organocatalytic asymmetric reactions where involving preformed sulfonium ylides and sulfoxonium ylides as reactants, but the reactions where the sulfur ylides participated in the transformations are generated in-situ from the corresponding salt precursors under basic conditions are not discussed[30-33]. Nevertheless, the related asymmetric reactions with chiral sulfide-centric catalysts involving chiral sulfur ylide intermediates[34-39] and those with chiral auxiliary-derived sulfonium salts as reactants are also not discussed here. This arrangement is mainly based on the consideration that those reactions were well described in previous elegant reviews[15,16,40,41].
ORGANOCATALYTIC ASYMMETRIC REACTION OF SULFONIUM YLIDES
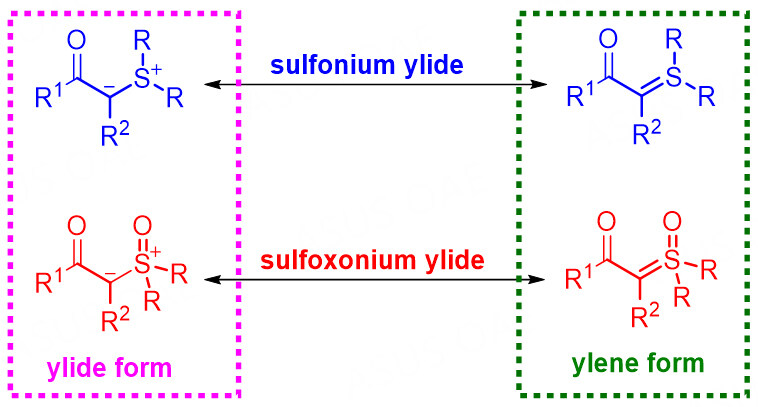
Sulfonium ylides can be represented by ylide form which contains a carbanion connecting a positively charged sulfur (IV) atom, or by ylene form which contains a C = S(IV) double bond [Figure 1]. The stability of sulfonium ylides depends largely on the degree of electron delocalization on the carbanion center. Therefore, stabilized sulfonium ylides generally bear an electron-withdrawing functional group connected to the carbanion center; thus, these ylides are readily prepared and usually used in various reactions. The stabilized -carbonyl sulfonium ylides can be applied as one-carbon synthons to construct C-X (X = C, N, S) bonds[17,40,42]. Moreover, they are more commonly used in cyclization reactions with electron-poor π-systems for the synthesis of various ring compounds[20,22]. In the area of organocatalysis, stabilized sulfonium ylides have been used in N-H bond insertion reactions, cyclopropanation reactions, and [4 + 1] cyclization reactions under the promotion from various organocatalyst via different activation patterns.
Asymmetric N-H bond insertion reaction
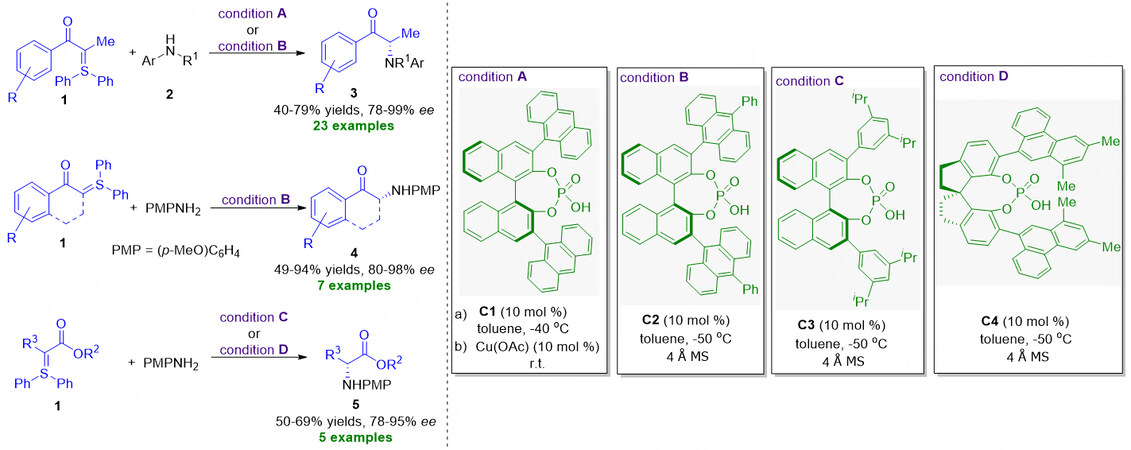
Chiral organocatalysts promoted asymmetric heteroatom-H bond insertion reaction with the sulfonium ylides remained highly challenging in the past two decades, and successful research was virtually nonexistent. Until 2020, Guo et al. disclosed the asymmetric N-H bond insertion reactions by -carbonyl sulfonium ylides using chiral phosphoric acids as catalysts, affording a series of chiral -amino ketone compounds with excellent efficiency and enantioselectivity [Figure 3][43]. Under conditions A or B, the transformation with -methyl sulfonium ylides 1 as substrates proceeded smoothly to form α-tertiary amino ketones 3 in 40%-79% yields with 78%-99% ee. As for the α-non-methyl sulfonium ylide or cyclic sulfonium ylide substrates, the reaction with condition B could give the corresponding α-amino ketones 4 bearing various alkyl chains or Cyclic α-amino ketones in 49%-94% yields with 80%-98% ee. In addition, α-alkyl esters derived sulfonium ylides 1 could also act as available starting materials to participate in the N-H bond insertion reaction with condition C or D, generating the desired α-amino esters 5 in excellent results (50%-69% yields, 78%-95% ee).
Figure 3. Asymmetric N-H bond insertion reaction by α-carbonyl sulfonium ylides. This figure is used with permission from the American Chemical Society[43].
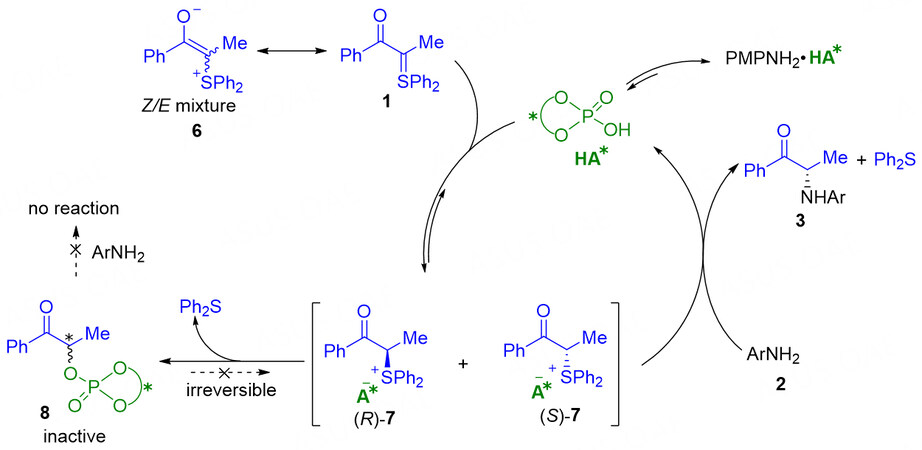
Moreover, the authors also investigated the possible mechanism. The mechanistic studies revealed that the reaction undergoes a protonation-amination sequence and the enantioselectivity is controlled by dynamic kinetic resolution in the amination step, rather than the initial protonation[43]. In the proposed mechanism [Figure 4], the chiral phosphoric acid enables the protonation of sulfonium ylide 1 that is in resonance with the enolate mixture of Z/E-isomers 6, to give an intermediate mixture of (S)-7 and (R)-7 that pair with chiral phosphate anion. Subsequent amine substitution by 2 delivers the desired chiral α-amino product 3 and simultaneously releases the chiral phosphoric acid catalyst. Indeed, intermediate 7 is in the catalyst resting state and permits sufficient epimerization of the labile tertiary stereocenter in (S)-7 and (R)-7. Thus, the enantioselectivity is controlled during amination by pseudo dynamic kinetic resolution of the intermediate mixture of (S)-7 and (R)-7. In addition, phosphate ester 8 as a by-product could be found in the catalytic mixture but is unable to react with amine 2 due to its inactiveness.
Figure 4. Proposed mechanism for chiral phosphoric acid catalyzed asymmetric N-H bond insertion reaction by α-carbonyl sulfonium ylides. This figure is used with permission from the American Chemical Society[43].
Asymmetric cyclization reaction of sulfonium ylides
Intermolecular cycloaddition is one of the most essential and powerful tools for the construction of cyclic skeletons. Indeed, since the significant breakthrough of asymmetric organocatalysis in the early 2000s[26-29], organocatalyzed enantioselective cycloaddition reactions involving sulfonium ylides have been widely explored for the synthesis of diverse cyclic compounds. In this section, we divide the organocatalyzed asymmetric transformations of sulfonium ylides into two categories: cyclopropanation and [4 + 1] cyclization reaction for simplicity.
Organocatalyzed asymmetric cyclopropanation
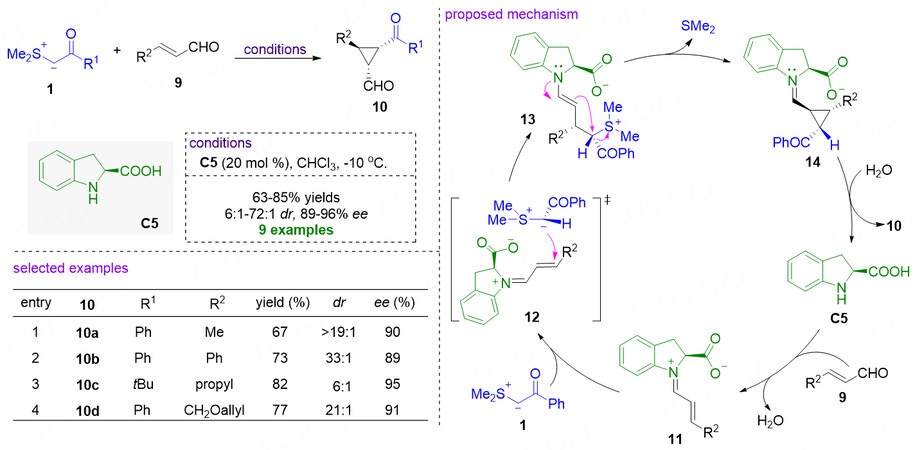
Since cyclopropanation of sulfonium ylides with electron-deficient olefins was developed by Corey and Chaykovsky in 1965[13], the research on the corresponding asymmetric version has been devoted continuous attention from chemists, and many great achievements have been made in this aspect[20]. The first enantioselective organocatalytic cyclopropanation was achieved by means of iminium activation in 2005
Figure 5. Chiral 2-carboxylic acid dihydroindole catalyzed enantioselective cyclopropanations of α,β-unsaturated aldehydes. This figure is used with permission from the American Chemical Society[44].
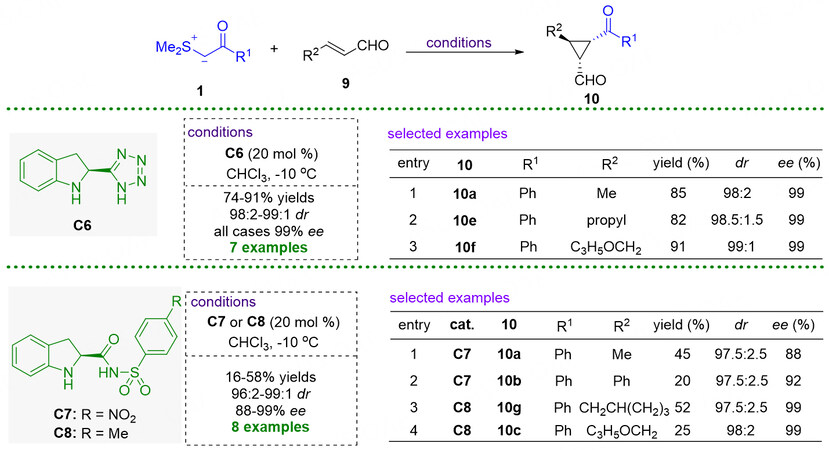
In 2007, Hartikka et al. also described the enantioselective cyclopropanation of α,β-unsaturated aldehydes with sulfonium ylides by using newly developed chiral amine catalysts [Figure 6][47,48]. With the tetrazolic acid functionalized dihydroindol C6 as the catalyst, chiral cyclopropanes 10 could be obtained in 99% ee for the seven examples[29]. In the presence of C7 or C8, the reactions gave the products with moderate yields with good to excellent diastereo- and enantioselectivities[30]. In their work, it was demonstrated that the larger steric bulk catalysts could increase enantioselective induction, thus leading to better enantioselectivities compared with the results obtained in Kunz and MacMillan’s work[44].
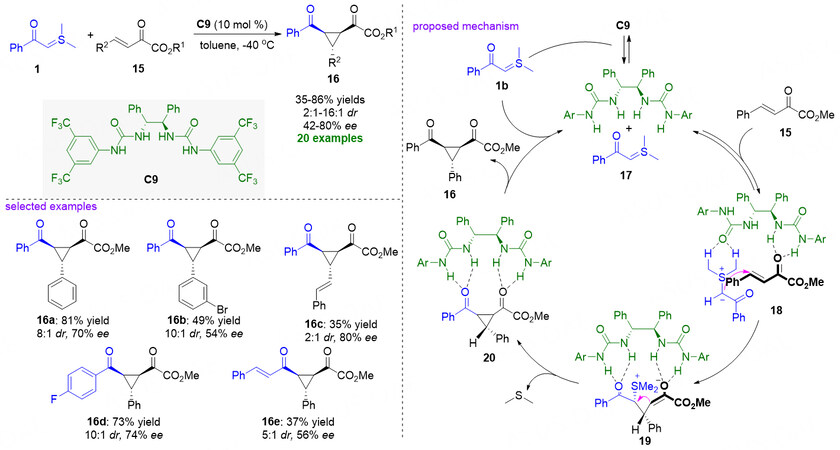
In the area of asymmetric organocatalysis, asymmetric hydrogen-bonding catalysis, which takes advantage of the hydrogen bonding interactions between the chiral catalysts and reactants to promote organic transformation and concurrently induce the stereoselectivity through lowering the LUMO of electrophiles, occupies a very important position[49-51]. In 2011, Cheng et al. employed C2-symmetric urea as a hydrogen-bond catalyst for the asymmetric cyclopropanation of β,γ-unsaturated α-ketoesters 15 with stabilized sulfonium ylides 1, providing a series of optically pure 1,2,3-trisubstituted cyclopropane derivatives 16 in moderate to good yields, diastereoselectivities, and enantioselectivities [Figure 7][52]. 1H NMR spectroscopic studies were conducted to demonstrate that the existence of H-bond interaction between C2-symmetric urea C9 and sulfonium ylide 1 could form complex 17. In the proposed pathway, complex 17 incorporates with β,γ-unsaturated α-ketoester 15 to form transition state 18. Then sulfonium ylide attacks the γ-position of ketoester to generate intermediate 19. Finally, intermediate 19 undergoes an intramolecular nucleophilic substitution reaction to give intermediate 20 that delivers corresponding chiral cyclopropane derivatives 16 and releases the hydrogen-bond urea catalyst for the next catalytic cycle.
Figure 7. Chiral C2-symmetric urea-catalyzed cyclopropanation of β,γ-unsaturated α-ketoesters. This figure is used with permission from the American Chemical Society[52].
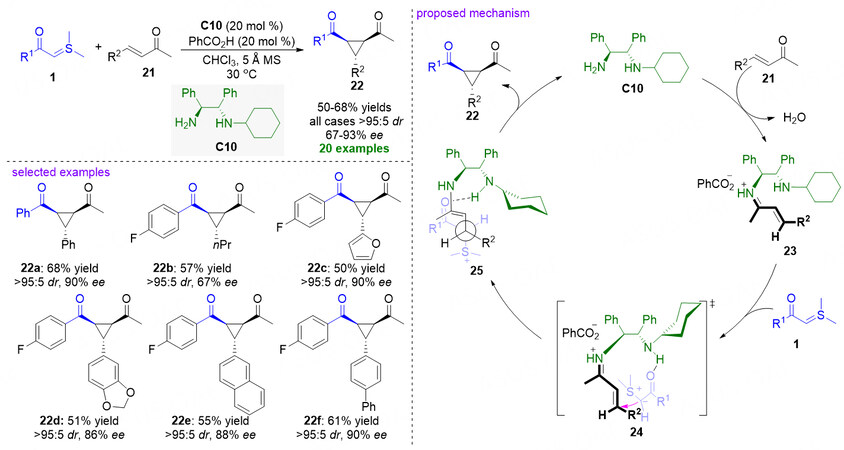
Until 2013, organocatalyzed asymmetric cyclopropanation of α,β-unsaturated ketone substrates with stabilized sulfonium ylides was reported by Wang et al.[Figure 8][53]. In the developed method, the primary amine part of chiral diamine catalyst C10 combines with α,β-unsaturated ketones 21 to form the iminium ion intermediates 23. Simultaneously, the secondary amine moiety of C10 incorporates with sulfonium ylides 1 through H-bond interaction. Then a Michael addition reaction is then performed from sulfonium ylides 1 to the Si face of iminium ion moiety, leading to the formation of intermediates 25. Subsequently, an intramolecular cyclization generates the desired cyclopropanation adducts 22 and releases the catalyst into the nest cycle.
Figure 8. Chiral diamine-catalyzed asymmetric cyclopropanation of α,β-unsaturated ketones. This figure is used with permission from the American Chemical Society[53].
Organocatalyzed asymmetric [4 + 1] cyclization reactions
There have been many reports on catalytic asymmetric [4 + 1] cyclization of sulfonium ylides for the construction of diverse five-membered cycles by using sulfide-centric catalysis, transition-metal catalysis, and organocatalysis. In particular, the organocatalysis strategy in this research area has gained increasing interest from the synthetic community due to its sustainable and green features.
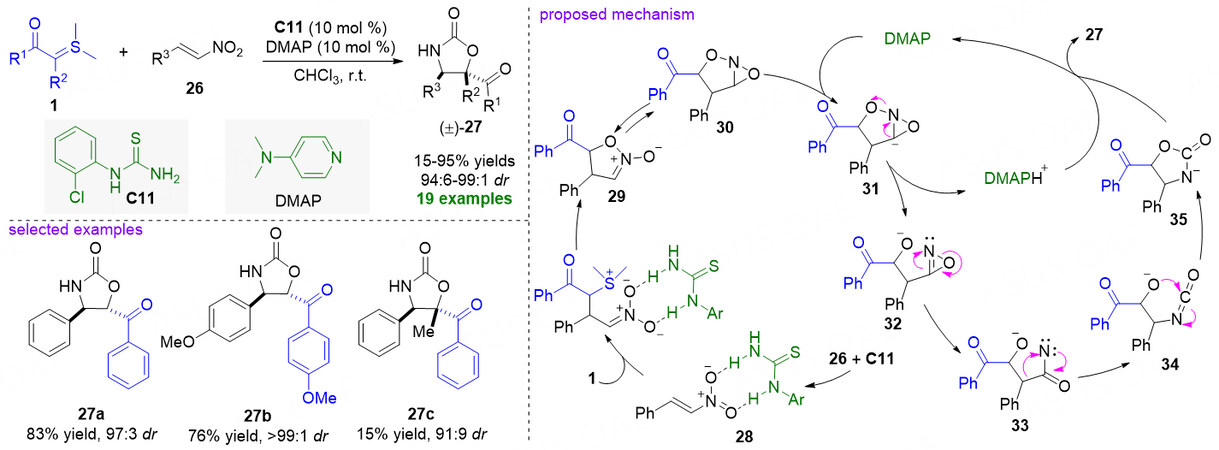
In 2008, Lu et al. reported an organocatalytic [4 + 1] cyclization reaction of nitroolefins 26 and sulfonium ylides 1 under ortho-chlorophenyl thiourea C11 and N,N-dimethylaminopyridine (DMAP) cooperative catalysis, allowing efficient and rapid access to diverse and structurally complex oxazolidin-2-ones (±)-27
Figure 9. ortho-chlorophenyl thiourea/DMAP cooperative catalysis for the [4 + 1] cyclization reaction of nitroolefins and stable sulfonium ylides. This figure is used with permission from the American Chemical Society[54].
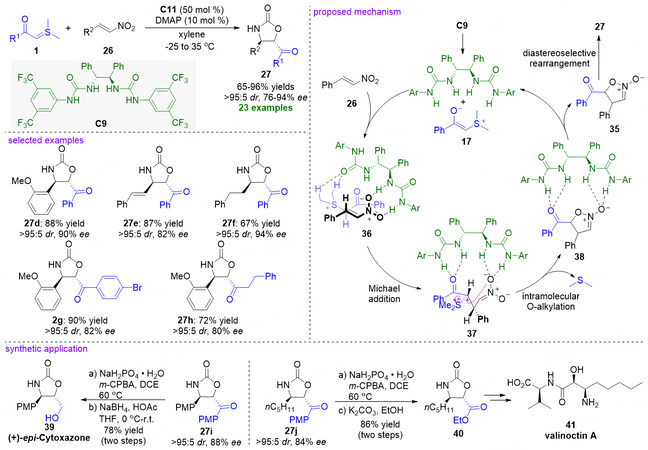
Impressively, the catalytic asymmetric version of the above [4 + 1] cyclization reaction between nitroolefins 26 and sulfonium ylides 1 was realized by the same group in 2012 [Figure 10][55]. The chiral C2-symmetric multiple hydrogen-bonding urea catalyst C9 shows the best positive effect on enantioselectivity and the method displays a broad substrate scope to give optically active oxazolidin-2-ones 27 in good to excellent yields and stereoselectivities (65%-96% yields, > 95:5 dr, and 76%-94% ee). The authors think the formation of chiral oxazolidin-2-ones depends largely on asymmetric [4 + 1] cyclization reaction, including a cascade Michael addition/intramolecular O-alkylation/rearrangement process. In addition, stepwise operation at temperature could ensure the high enantioselectivity of the reaction. Moreover, synthetic applications were also conducted. (+)-epi-cytoxazone 39[56] and key intermediate 40 for synthesis of the valinoctin A 41[57] were respectively provided after two steps.
Figure 10. Catalytic asymmetric [4 + 1] cyclization reaction of nitroolefins and stabilized sulfonium ylides. This figure is used with permission from the Wiley-VCH Verlag[55].
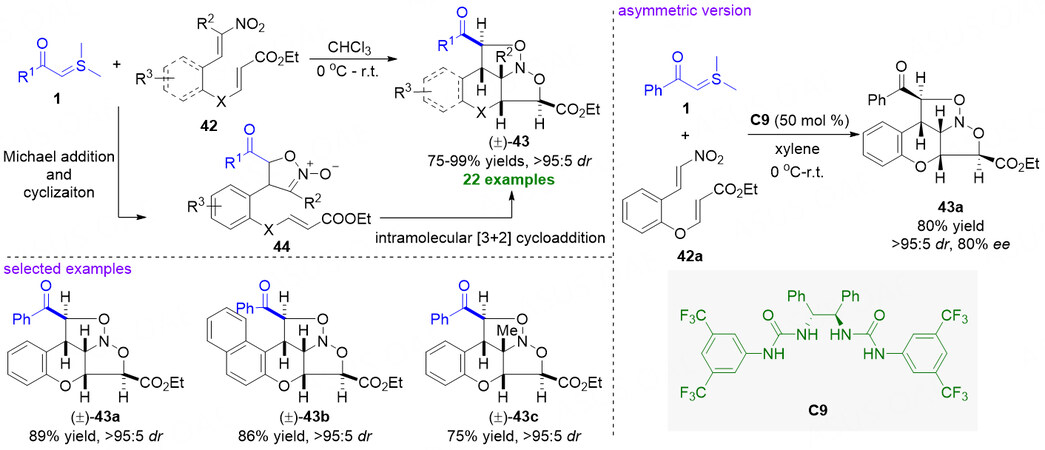
Based on their successful implementation of thiourea/DMAP cooperative-catalysis strategy for the diastereoselective [4 + 1] cyclization reaction of nitroolefins and sulfonium ylides[54], Xiao and coworkers became interested in the application of the vital isoxazoline N-oxides 29 [Figure 9] intermediate as a type of 1,3-dipole species for the possible further reactions. In 2009, Lu et al. reported a catalyst-free sequential intermolecular [4 + 1] and intramolecular [3 + 2] cycloaddition reaction between sulfonium ylides 1 and alkene-tethered nitroolefins 42 [Figure 11][58]. The protocol exhibits a wide substrate scope and excellent diastereocontrol, but also smoothly delivers a range of important N-containing polycyclic compounds (±)-43 bearing five consecutive stereogenic centers in good to high yields (75%-99%) with excellent diastereoselectivities (all cases > 95:5 dr). In the cascade process, sulfonium ylides 1 and alkene-tethered nitroolefins 42 firstly undergo a Michael addition and cyclization to form an important intermediate 44, which quickly converts to products 43 through an intramolecular [3 + 2] cycloaddition. In addition, asymmetric synthesis of the polycyclic product was also investigated. Selecting chiral C2-symmetric urea C9 as multiple hydrogen bond catalyst, the reaction proceeded smoothly and afforded chiral product 43a in 80% yield with > 95:5 dr and 80% ee.
Figure 11. Formal [4 + 1]/[3 + 2] cycloaddition cascade of sulfonium ylides and alkene-tethered nitroolefins. This figure is used with permission from the Wiley-VCH Verlag[58].
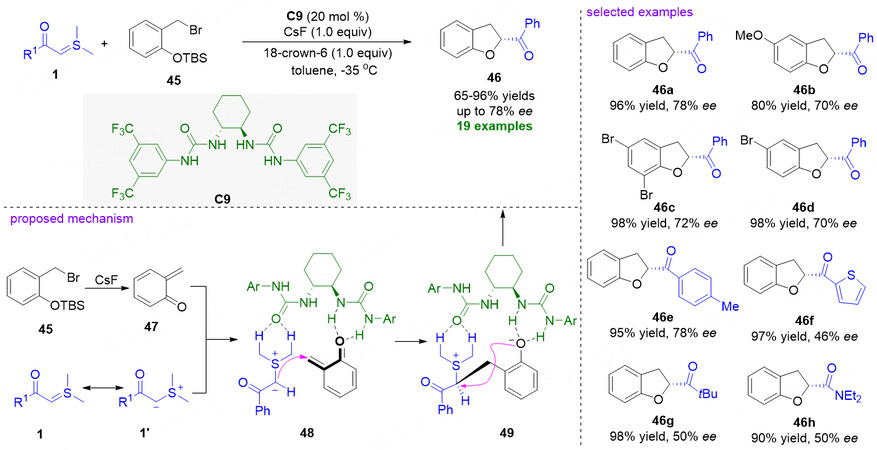
Chiral 2,3-dihydrobenzofurans are ubiquitous and important ring structures embedded in some natural products and synthetic drugs with diverse bioactivities. Consequently, the synthesis of structurally diverse 2,3-dihydrobenzofuran compounds via a catalytic asymmetric manner has attracted tremendous attention from synthetic chemists[59,60]. In 2015, Lei et al. reported a facile synthesis of racemic 2,3-dihydrobenzofurans via formal [4 + 1] cyclization of sulfonium ylides and in situ generated ortho-quinone methides (o-QMs) from O-silylated phenols[61]. Two years later, Yang and Xiao developed a catalytic asymmetric version of the same reaction as reported by Sun and Xu, using the chiral C2-symmetric multiple hydrogen-bonding urea catalyst C9 [Figure 12][62]. The developed asymmetric catalytic protocol exhibits a broad substrate scope and high functional group tolerance. A wide range of chiral 2,3-dihydrobenzofurans could be obtained in good to excellent yields with moderate stereoselectivities. In the proposed reaction mechanism, the organocatalyst C9 simultaneously interacts with sulfonium ylide 1 and the in situ generated ortho-quinone methide 47 by H-bonding interactions to form complex 48. Subsequently, asymmetric Michael addition leads to the formation of complex 49, which then undergoes an intramolecular SN2 displacement to deliver chiral 2,3-dihydrobenzofuran products 46.
Figure 12. Catalytic asymmetric [4 + 1] cyclization reaction of sulfonium ylides with the in situ generated ortho-quinone methides. This figure is used with permission from the Wiley-VCH Verlag[62].
ORGANOCATALYTIC ASYMMETRIC REACTION OF SULFOXONIUM YLIDES
Compared to sulfonium ylides, sulfoxonium ylides contain a positively charged sulfur(VI) atom in the ylide form or a C = S(VI) double bond in the ylene form due to the binding of one oxygen atom to the sulfur atom. Therefore, the sulfur oxidation state would result in some differences in the property and the reactivity for the ylides. The sulfoxonium ylides are relatively more stable and have lower nucleophilicity. In addition, the electronegative oxygen atom readily coordinates with a chiral Lewis acid catalyst or combines with the hydrogen bond of organocatalysts, thereby enhancing the stereoselectivity control in asymmetric catalysis. Consequently, in the past three decades, many elegant implementations of sulfoxonium ylides in organic synthesis have been reported by many research communities and those in the industry[17,23,24]. However, probably due to the relatively less reactive and low nucleophilicity of carbanion by comparing with their sulfonium ylide counterparts, significant progress on sulfoxonium ylides-involved asymmetric reaction by organocatalysis is far lagging behind, although transition-metal catalytic asymmetric transformations were reported from 2007[63-67]. Likewise, in this section, the organocatalytic asymmetric reaction of sulfoxonium ylides will also be discussed in two parts, including the X-H (X = S, N, C) bond insertion reaction and cyclization reaction.
Asymmetric X-H (X = S, N, C) bond insertion reaction
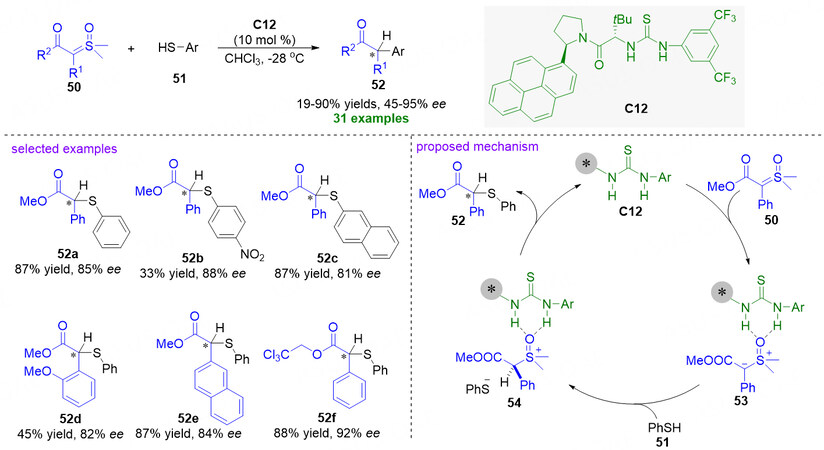
In 2016, Burtoloso and coworkers reported a catalyst-free S-H bond insertion reaction of sulfoxonium ylides into aryl thiols, which exhibits a broad substrate scope and high yields for the direct synthesis of β-keto thioethers under mild reaction conditions[68]. By the year 2020, Burtoloso, Mattson and co-workers realized an enantioselective S-H bond insertion reaction of α-carbonyl sulfoxonium ylides to a diverse array of aryl thiols by taking advantage of a known thiourea catalyst C12 to control the enantioselectivity
Figure 13. Chiral thiourea catalyzed enantioselective S-H insertion reactions of α-carbonyl sulfoxonium ylides to aryl thiols. This figure is used with permission from the Wiley-VCH Verlag[69].
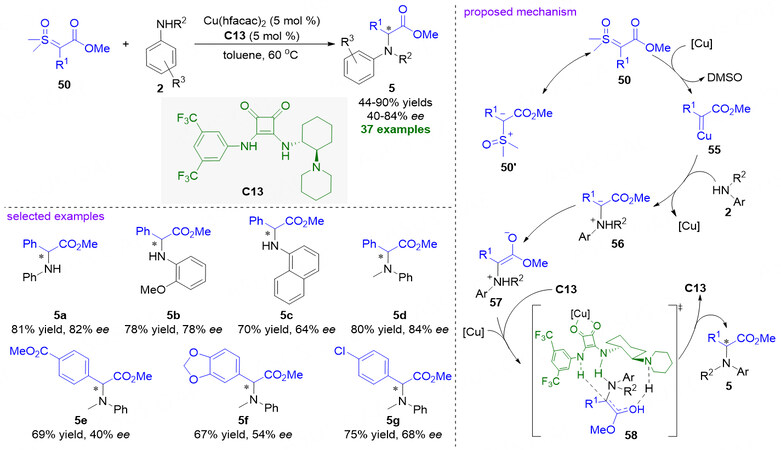
α-Amino esters as versatile building blocks have significant applications in organic synthesis as well as widespread distribution in complex natural products, biomolecules and clinically used drugs[70]. Thereby remarkable achievements have been made in the development of various asymmetric methods for the construction of optically pure α-imino esters[71]. Indeed, the asymmetric N-H insertion reactions of α-carbonyl sulfoxonium ylides to amines are able to provide direct access to chiral α-amino esters. In 2021, Furniel et al. reported the first asymmetric reaction of α-carbonyl sulfoxonium ylides and anilines by using a copper-bifunctional squaramide cooperative catalysis strategy, which undergoes an asymmetric N-H insertion process to afford a series of chiral α-aryl glycine esters 5 in good to excellent yields and enantioselectivities ranging between 40%-84% ee [Figure 14][72]. A mechanism proposal was also proposed. At first, the nucleophilic attack of sulfoxonium ylide 50 to copper complex generates copper carbene intermediate 55, which could transform into ammonium ylide intermediate 56 by the attack of aniline 2. And then, the intermediate 56 tautomerizes to the enol intermediate 57, which readily interacts with copper-chiral squaramide complex through hydrogen bond to form transition state 58. Finally, under asymmetric induction, protonation of the enol intermediate 57 leads to the formation of enantioenriched N-H insertion products α-aryl glycine esters 5.
Figure 14. Cooperative copper-bifunctional squaramide catalysis for the asymmetric N-H insertion reactions of α-carbonyl sulfoxonium ylides and anilines. This figure is used with permission from the Royal Society of Chemistry[72].
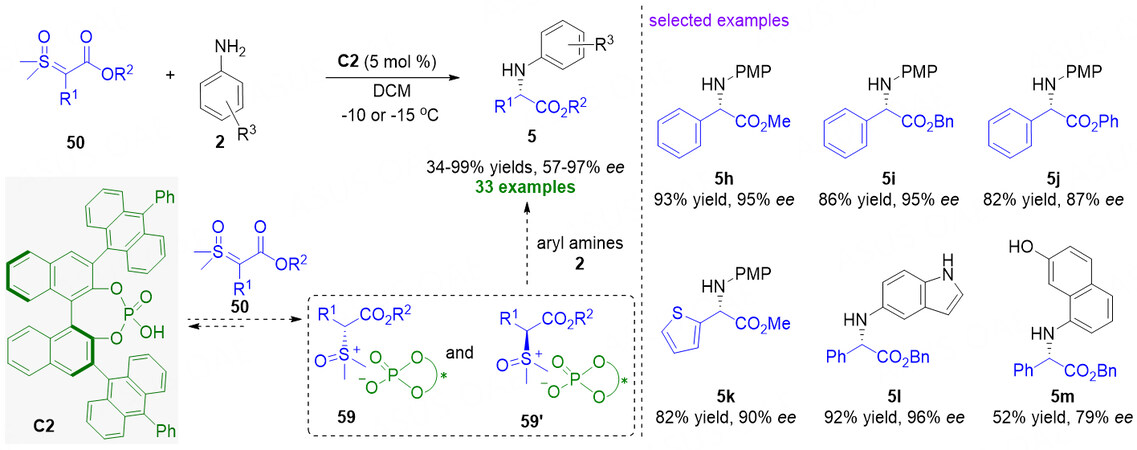
Almost at the same time, Guo et al. also reported a chiral phosphoric acid-catalyzed asymmetric N-H insertion reaction of aryl amines 2 with α-carbonyl sulfoxonium ylides 50 [Figure 15][73]. With bulky chiral phosphoric acid C2 as the catalyst, a wide range of α-aryl glycines were obtained in generally excellent yields and enantiomeric excesses (34%-99% yield and 57%-97% ee). In the proposed mechanism, the weak basicity of sulfoxonium ylides could cause the interaction with chiral phosphoric acid C2 to form the ion pair intermediates 59 and 59', which undergo nucleophilic attack by the aryl amines 2, thus leading to the formation of α-aryl glycine esters 5. After detailed mechanistic studies, the authors think that the protonation of 50 favors the formation of intermediates 59 and 59'. Due to reversiblity of this step, the chiral phosphate anion can control the stereochemistry in the C-N bond formation via dynamic kinetic resolution. The C-N bond formation is also rate-determining step.
Figure 15. Chiral phosphoric acid-catalyzed enantioselective N-H insertion reactions of α-carbonyl sulfoxonium ylides. This figure is used with permission from the Royal Society of Chemistry[73].
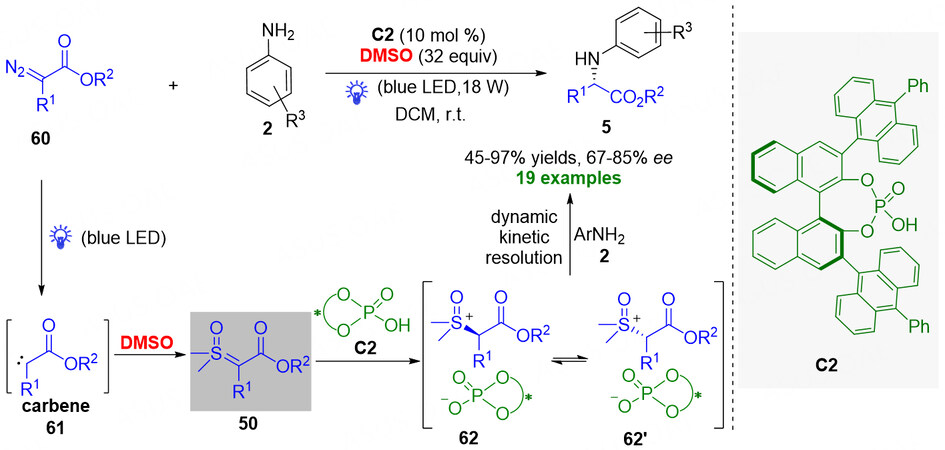
Although that catalytic asymmetric insertion reaction of metal carbenes into N-H bond with the enantioselective control via metal-chiral metal interaction has been well-established[74,75], the asymmetric insertion reaction of free carbenes into N-H bond faces a challenge due to strong background reactions and lack of any anchor for a catalyst interaction. Recently, Guo et al. made progress in the catalytic enantioselective insertion reaction of free carbenes into N-H bond using an indirect approach[76]. In their developed protocol [Figure 16], with visible light as a promoter, the -diazoesters 60 could be converted to free carbene intermediate 61, which was captured rapidly by DMSO to in situ form sulfoxonium ylide 50. And then, chiral phosphoric acid catalyst C2 protonated ylide 50 to generate diastereomeric intermediates 62 and 62'. Ultimately, under the control of a chiral catalyst, the amine reactants 2 reacted preferentially with one of the intermediates 62 and 62' to deliver a range of optically pure α-aminoesters with high efficiency (45%-97% yield) and good enantioselectivity (67%-85% ee) through a dynamic kinetic resolution process. Moreover, the authors declared that the key to the success of the method lay in the formation of sulfoxonium ylide by the additive DMSO capturing the free carbene intermediate.
Figure 16. Visible-light-induced organocatalytic enantioselective N-H insertion of α-diazoesters with in situ generated sulfoxonium ylides. This figure is used with permission from the Royal Society of Chemistry[76].
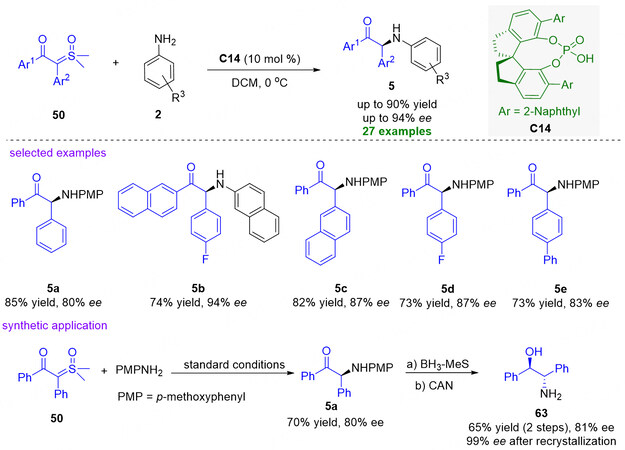
Almost immediately, the same group also discovered an efficient organocatalytic formal N-H insertion reaction of α-keto sulfoxonium ylides 50 bearing two aryl groups and arylamines 2 using chiral phosphoric acid C14 as catalyst [Figure 17][77]. With the developed protocol, a broad range of α-tertiary aminoketones 5 could be smoothly obtained in good to excellent yields (up to 90%) and ee values (up to 94%). Their mechanistic study supported that the C-N bond formation via dynamic kinetic resolution is not only the rate-determining step but also the enantio-determining step. In addition, the synthetic utility of the method was also demonstrated by the preparation of 2-amino-1,2-diphenylethanol 63 in 65% yield with 81% ee through a two-step transformation from 5a. More delightfully, enantiopure 63 could be readily obtained by single recrystallization in 60% yield.
Figure 17. Organocatalytic formal N-H insertion reaction of arylamines with α-keto sulfoxonium ylides bearing two aryl groups. This figure is used with permission from the Royal Society of Chemistry[77].
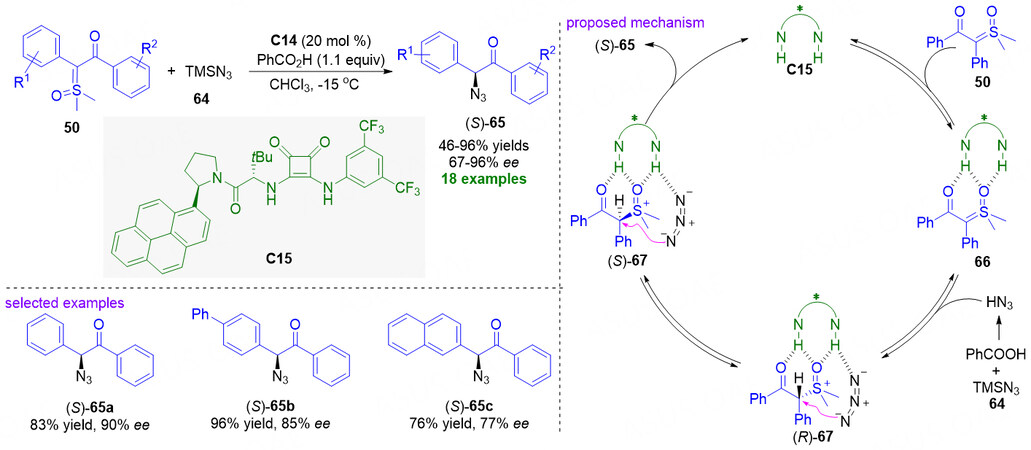
Very recently, Guo et al. discovered an organocatalytic asymmetric azidation of α-carbonyl sulfoxonium ylides through a H-N3 insertion process [Figure 18][78]. In the developed method, with the in situ formed HN3 as nitrogen source and a superb chiral squaramide C15 as the catalyst, a series of α-carbonyl sulfoxonium ylides 50 could be smoothly converted into optically active α-azido ketones 65 in 46%-96% yield with 67%-96% ee. In the proposed mechanism, the catalyst C15 with the double N-H motifs activates sulfoxonium ylides 50, giving intermediate 66. And then, the in situ generated HN3 promotes the protonation of intermediate 66 to form ion pair intermediate 67 as diastereomeric mixture, in which the azide anion also interacts with the squaramide catalyst through hydrogen bond. Notably, the reversible protonation process in the 66 to 67 step allows (R)-67 and (S)-67 to rapidly equilibrate with each other, resulting in the epimerization of the stereogenic center. Finally, the enantioenriched (S)-65 could be preferentially generated by the azide substitution via dynamic kinetic resolution process under the stereoselective control of chiral squaramide catalyst C15.
Figure 18. Chiral squaramide-catalyzed enantioselective H-N3 insertion of α-carbonyl sulfoxonium ylides. This figure is used with permission from the Royal Society of Chemistry[78].
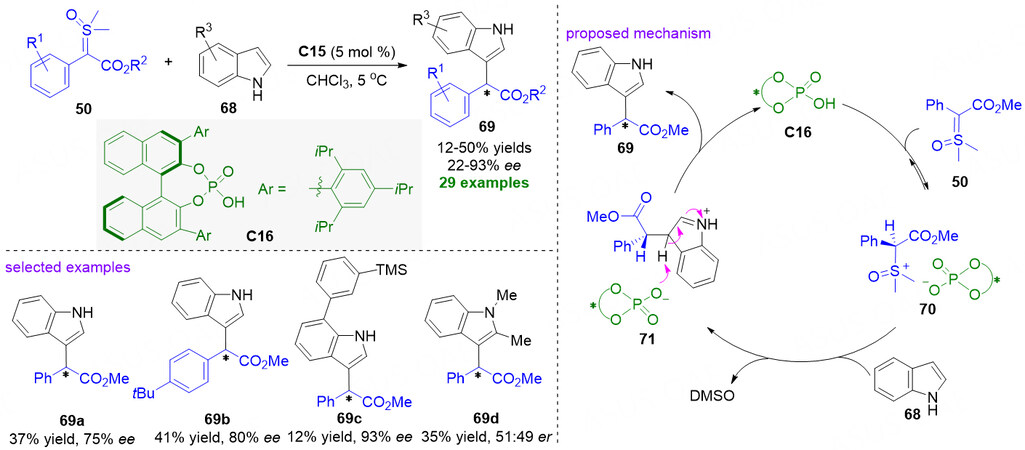
After the catalytic asymmetric S-H, N-H insertion reaction of sulfoxonium ylides, Leveille et al. recently reported the first organocatalytic enantioselective C-H insertion reaction of sulfoxonium ylides to indoles with chiral phosphoric acid as the catalyst 2021 [Figure 19][79]. The authors investigated 29 examples using α-carbonyl sulfoxonium ylides insertion into C-H bond of free indoles, obtaining moderate yields (ranging from 12% to 50%) with 20%-93% ee values. A proposed pathway for the C-H insertion reaction was also introduced. Initially, chiral phosphoric acid C16 protonates the α-carbonyl sulfoxonium ylides 50 to form an ion pair intermediate 70. And then, the indole partners attack the intermediate 70 via a substitution reaction under the enantioselective induction of chiral phosphoric acid catalyst to give rise to the chiral product 69, simultaneously reprotonation of catalyst ion to release C15 into the next cycle. Notably, the N-H moiety of indole 68 is crucial to enantioselectivity in this transformation. The reaction involving N-Me indole proceeded smoothly to furnish product 69d in 35% yield, but essentially no enantiomeric excess was observed.
Figure 19. Chiral phosphoric acid-catalyzed C-H insertion reactions of α-carbonyl sulfoxonium ylides to indoles. This figure is used with permission from the American Chemical Society[79].
Asymmetric cyclization reaction of sulfoxonium ylides
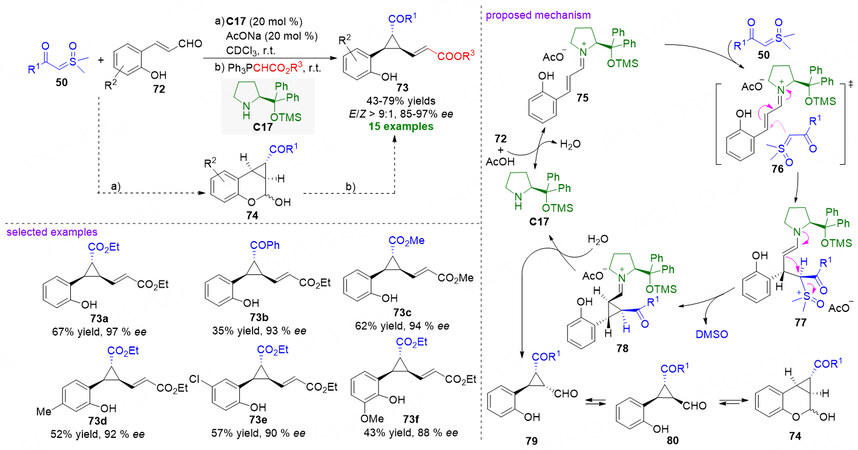
Similarly to sulfonium ylides, in addition to the participation in the above-mentioned catalytic asymmetric X-H (X = S, N, C) bond insertion reactions, sulfoxonium ylides can also be employed as nucleophilic 1C synthons for the cyclization reaction to access cyclic compounds. However, the asymmetric cyclization reaction involving sulfoxonium ylides by means of an organocatalysis tactic is rarely studied. Until recently, Bernardi, Fochi, and coworkers reported the first organocatalytic tandem cyclopropanation/hemiaminalization reaction between 2’-hydroxycinnamaldehydes 72 and stabilized α-carbonyl sulfoxonium ylides 50 by using Jørgensen-Hayashi catalyst C17, providing cyclopropane-fused chromane derivatives 74 in moderate-to-good yields with very good stereocontrol. Moreover, after a Wittig olefination to the chromanol moiety of 70, the tricyclic ring-fused compounds 74 could be further transformed into a series of cyclopropanation products 73 [Figure 20][80].
Figure 20. Organocatalytic asymmetric tandem cyclopropanation/hemiaminalization reaction of 2’-hydroxycinnamaldehydes and stabilized α-carbonyl sulfoxonium ylides. This figure is used with permission from the American Chemical Society[80].
In the proposed reaction pathway [Figure 20], acid mediated condensation of 2’-hydroxycinnamaldehydes 72 and chiral catalyst C17 forms reactive iminium ion intermediate 75, which is attacked by the sulfoxonium ylides 50 via transition state 76 to furnish intermediate 77. And then, an intramolecular nucleophilic substitution reaction of 77 leads to the formation of iminium ion 78, which would undergo hydrolysis to release the catalyst C17 and to deliver 2,3-trans cyclopropane 79. Subsequent epimerization of the α-position to the aldehyde function would give rise to the generation of 2,3-cis-isomers 80, which undergo hemiacetalization to give cyclopropane-fused chromane products 74.
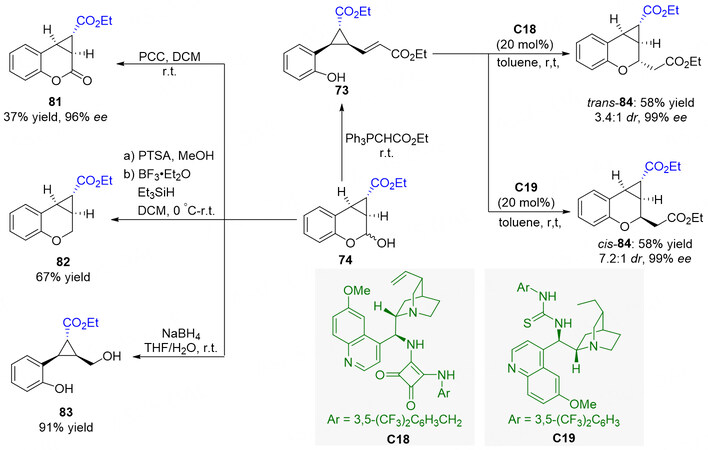
In addition, the synthetic applications of the protocol were also demonstrated by the versatile derivatizations of the cyclopropane-fused chromane 74 and the cyclopropanation product 73. As shown in Figure 21, the hemiacetal functionality of 74 can be leveraged as a synthetic handle enabling access to some other compounds. A treatment of 74 with PCC gives rise to the oxidization of the hemiacetal group to form compound 81 in moderate yield. 4 could be smoothly reduced to 82 in 67% yield by using triethylsilane in the presence of BF3•OEt, and could also be converted to 83 in 91% yield with NaBH4. Moreover, cyclopropanation product 73 could undergo intramolecular asymmetric oxa-Michael reaction. With Cinchona alkaloid-derived bifunctional catalysts C18 and C19 for the Michael addition, the tricyclic ring-fused products trans-84 and cis-84 could be obtained in moderate yield with high diastereoselectivity and excellent enantioselectivity, respectively.
Figure 21. Various derivatizations of cyclopropane-fused chromane 70 and the cyclopropanation product 69. This figure is used with permission from the American Chemical Society[80].
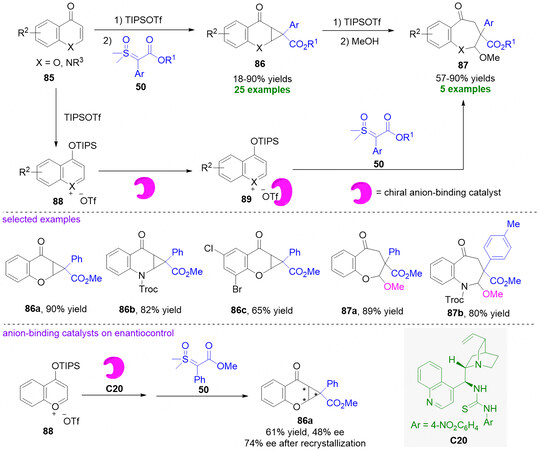
In 2022, Leveille et al. discovered that the benzopyrylium triflates 88 in situ generated from chromenones/quinolones 85 and triisopropylsilyl triflate (TIPSOTf) could react with sulfoxonium ylides 50 via a cyclopropylation process to access a range of cyclopropanated products 86 in 18%-90% yield[81]. Furthermore, the authors also converted several products 86 into benzo[b]oxepines 87 with 57%-90% yield via acid-mediated ring-expansion in the presence of a nucleophile [Figure 22][81]. In addition, the authors also conceived that the possibility of controlling the enantioselectivity of the cyclopropanation would be offered by a suitable chiral anion-binding catalyst through the non-covalent interaction with benzopyrylium triflates 88 for the formation of intermediate 89. Therefore, with a cinchonidine-derived thiourea C20 as the catalyst, the reaction of benzopyrylium triflate 88 and sulfoxonium ylide 50 gave rise to product 86a in 61% yield with 48% ee, which could be improved to 74% ee after simple recrystallization.
Figure 22. Dearomatization of in situ generated benzopyrylium triflates with sulfoxonium ylides. This figure is used with permission from the Royal Society of Chemistry[81].
CONCLUSION AND OUTLOOK
Sulfur-based ylides have been identified and confirmed as versatile reactants for a wide range of chemical transformations. In this review, organocatalyzed asymmetric reactions involving sulfonium and sulfoxonium ylides are summarized and reviewed. Due to the eminent characteristics of stabilized sulfonium and sulfoxonium ylides, such as thermal stability, easy preparation, storage, and use, and low toxicity, these compounds have drawn great attention from many research groups and are widely used in various catalytic asymmetric reactions over the past twenty years with the rapid development of organocatalysis. As to the research on sulfonium ylides, the organocatalyzed enantioselective transformations mainly focus on the cyclopropanation and [4 + 1] cyclization reaction, and only one report concerning the N-H bond insertion reaction has been published so far. However, with respect to the sulfoxonium ylides, synthetic chemists put relatively more research emphasis on the catalytic asymmetric X-H (X = S, N, C) bond insertion reaction for the C-C and C-X (X = N, S) bond construction, and only one example about asymmetric cyclization reaction has been reported with organocatalysis at present.
Although a number of reports are available on the application of sulfonium and sulfoxonium ylides in various asymmetric reactions, there is still a vast space for research on the fast-developing asymmetric organocatalysis. First of all, since the stabilized sulfur ylides are still limited, more and more newly stabilized sulfonium or sulfoxonium ylides with more fascinating skeletons should be explored. Second, searching for a new catalytic system for the application of unstabilized sulfur ylides in the possible asymmetric reaction should also be investigated. Third, expanding the scopes and sources of reaction partners with sulfur ylides to develop novel asymmetric reactions for the construction of pharmaceutically valuable molecules with innovative structures is very necessary. Finally, applying the developed asymmetric organocatalysis methodology involving sulfonium or sulfoxonium ylides for the efficient synthesis of natural products or pharmaceutical molecules is also worth pursuing. The main purpose of this review is to disclose the current situation of organocatalyzed asymmetric reaction of sulfnoium and sulfoxonium ylides, but we would rather it will provoke further exciting, groundbreaking discoveries soon. In a word, the authors hope this review in this phase benefits the development of sulfur ylides research area. Moreover, the authors would like to apologize in advance for any omissions in the literature survey.
DECLARATIONS
AcknowledgmentsWe sincerely thank all leading chemists and coworkers involved in the development of organocatalyzed asymmetric reaction of sulfnoium and sulfoxonium ylides.
Authors’ contributionsWrote the draft manuscript: Wang ZH
Revised the manuscript: Sun TJ, Zhang YP, You Y, Zhao JQ, Yin JQ
Guided this work, revised and rewrote some parts of the manuscript: Yuan WC
Availability of data and materialsNot applicable.
Financial support and sponsorshipWe are grateful to the Natural Science Foundation of China (No. 22171029, 21801024, 21901024, 21871252, 21801026, and 22271027), the Sichuan Science and Technology Program (2021YFS0315).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Wang N, Saidhareddy P, Jiang X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat Prod Rep 2020;37:246-75.
2. Petkowski JJ, Bains W, Seager S. Natural products containing a nitrogen-sulfur bond. J Nat Prod 2018;81:423-46.
3. Ferro CT, Dos Santos BF, da Silva CDG, Brand G, da Silva BAL, de Campos Domingues NL. Review of the syntheses and activities of some sulfur-containing drugs. Curr Org Synth 2020;17:192-210.
4. Otocka S, Kwiatkowska M, Madalińska L, Kiełbasiński P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis. Chem Rev 2017;117:4147-81.
5. Carreño MC, Hernández-Torres G, Ribagorda M, Urbano A. Enantiopure sulfoxides: recent applications in asymmetric synthesis. Chem Commun 2009:6129-44.
6. Trost BM, Rao M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew Chem Int Ed Engl 2015;54:5026-43.
7. Colomer I, Velado M, Fernández de la Pradilla R, Viso A. From allylic sulfoxides to allylic sulfenates: fifty years of a never-ending [2,3]-sigmatropic rearrangement. Chem Rev 2017;117:14201-43.
8. Chauhan P, Mahajan S, Enders D. Organocatalytic carbon-sulfur bond-forming reactions. Chem Rev 2014;114:8807-64.
9. Ingold CK, Jessop JA. XCV.-Influence of poles and polar linkings on the course pursued by elimination reactions. Part IX. Isolation of a substance believed to contain a semipolar double linking with participating carbon. J Chem Soc 1930;0:713-8.
10. Johnson AW, Lacount RB. The chemistry of ylids. VI. Dimethylsulfonium fluorenylide - a synthesis of epoxides. J Am Chem Soc 1961;83:417-23.
11. Corey EJ, Chaykovsky M. Dimethylsulfonium methylide, a reagent for selective oxirane synthesis from aldehydes and ketones. J Am Chem Soc 1962;84:3782-3.
12. Corey EJ, Chaykovsky M. Methylsulfinyl carbanion (CH3-SO-CH2-). Formation and applications to organic synthesis. J Am Chem Soc 1965;87:1345-53.
13. Corey EJ, Chaykovsky M. Dimethyloxosulfonium methylide ((CH3)2SOCH2) and dimethylsulfonium methylide ((CH3)2SCH2). Formation and application to organic synthesis. J Am Chem Soc 1965;87:1353-64.
15. McGarrigle EM, Myers EL, Illa O, Shaw MA, Riches SL, Aggarwal VK. Chalcogenides as organocatalysts. Chem Rev 2007;107:5841-83.
16. Sun XL, Tang Y. Ylide-initiated michael addition-cyclization reactions beyond cyclopropanes. Acc Chem Res 2008;41:937-48.
17. Burtoloso ACB, Dias RMP, Leonarczyk IA. Sulfoxonium and sulfonium ylides as diazocarbonyl equivalents in metal-catalyzed insertion reactions: sulfoxonium and sulfonium ylides in insertion reactions. Eur J Org Chem 2013;2013:5005-16.
18. Wu X, Sun S, Yu J, Cheng J. Recent applications of
19. Ushakov PY, Ioffe SL, Sukhorukov AY. Recent advances in the application of ylide-like species in [4 + 1]-annulation reactions: an updated review. Org Chem Front 2022;9:5358-82.
20. Lu LQ, Li TR, Wang Q, Xiao WJ. Beyond sulfide-centric catalysis: recent advances in the catalytic cyclization reactions of sulfur ylides. Chem Soc Rev 2017;46:4135-49.
21. Neuhaus JD, Oost R, Merad J, Maulide N. Sulfur-based ylides in transition-metal-catalysed processes. Top Curr Chem 2018;376:15.
22. Kaiser D, Klose I, Oost R, Neuhaus J, Maulide N. Bond-forming and -breaking reactions at sulfur(IV): sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem Rev 2019;119:8701-80.
23. Bisag GD, Ruggieri S, Fochi M, Bernardi L. Sulfoxonium ylides: simple compounds with chameleonic reactivity. Org Biomol Chem 2020;18:8793-809.
24. Caiuby CAD, Furniel LG, Burtoloso ACB. Asymmetric transformations from sulfoxonium ylides. Chem Sci 2022;13:1192-209.
25. Fan R, Tan C, Liu Y, et al. A leap forward in sulfonium salt and sulfur ylide chemistry. Chin Chem Lett 2021;32:299-312.
26. Xiang SH, Tan B. Advances in asymmetric organocatalysis over the last 10 years. Nat Commun 2020;11:3786.
27. Albrecht Ł, Albrecht A, Dell’Amico L. Asymmetric organocatalysis: new strategies, catalysts, and opportunities. Weinheim: Wiley-Vch Gmbh; 2023.
28. Mancheño O, Waser M. Recent developments and trends in asymmetric organocatalysis. Eur J Org Chem 2023;26:e202200950.
29. Ardevines S, Marqués-lópez E, Herrera RP. Horizons in asymmetric organocatalysis: en route to the sustainability and new applications. Catalysts 2022;12:101.
30. Biswas A, De Sarkar S, Tebben L, Studer A. Enantioselective cyclopropanation of enals by oxidative N-heterocyclic carbene catalysis. Chem Commun 2012;48:5190-2.
31. Li C, Jiang K, Liu T, Chen Y. Asymmetric [4 + 1] cycloadditions of
32. Akagawa K, Takigawa S, Nagamine IS, Umezawa R, Kudo K. Peptide-catalyzed diastereo- and enantioselective cyclopropanation of aromatic
33. Zhong C, Gautam LN, Petersen JL, Akhmedov NG, Shi X. Concise asymmetric synthesis of fully substituted isoxazoline-
34. Davoust M, Brière JF, Jaffrès PA, Metzner P. Design of sulfides with a locked conformation as promoters of catalytic and asymmetric sulfonium ylide epoxidation. J Org Chem 2005;70:4166-9.
35. Saito T, Sakairi M, Akiba D. Enantioselective synthesis of aziridines from imines and alkyl halides using a camphor-derived chiral sulfide mediator via the imino Corey-Chaykovsky reaction. Tetrahedron Lett 2001;42:5451-4.
36. Zanardi J, Leriverend C, Aubert D, Julienne K, Metzner P. A catalytic cycle for the asymmetric synthesis of epoxides using sulfur ylides. J Org Chem 2001;66:5620-3.
37. Minière S, Reboul V, Metzner P, Fochi M, Bonini BF. Catalytic ferrocenyl sulfides for the asymmetric transformation of aldehydes into epoxides. Tetrahedron Asymmetr 2004;15:3275-80.
38. Ishizaki M, Hoshino O. Synthesis of novel
39. Winn CL, Bellenie BR, Goodman JM. A highly enantioselective one-pot sulfur ylide epoxidation reaction. Tetrahedron Lett 2002;43:5427-30.
40. Aggarwal VK, Winn CL. Catalytic, asymmetric sulfur ylide-mediated epoxidation of carbonyl compounds: scope, selectivity, and applications in synthesis. Acc Chem Res 2004;37:611-20.
41. Li AH, Dai LX, Aggarwal VK. Asymmetric ylide reactions: epoxidation, cyclopropanation, aziridination, olefination, and rearrangement. Chem Rev 1997;97:2341-72.
42. Müller P, Fernandez D, Nury P, Rossier J. Transition-metal-catalyzed carbenoid reactions of sulfonium ylides. Helv Chim Acta 1999;82:935-45.
43. Guo W, Luo Y, Sung HH, Williams ID, Li P, Sun J. Chiral phosphoric acid catalyzed enantioselective synthesis of
44. Kunz RK, MacMillan DW. Enantioselective organocatalytic cyclopropanations. The identification of a new class of iminium catalyst based upon directed electrostatic activation. J Am Chem Soc 2005;127:3240-1.
45. Lakhdar S, Appel R, Mayr H. How does electrostatic activation control iminium-catalyzed cyclopropanations? Angew Chem Int Ed Engl 2009;48:5034-7.
46. Georgieva MK, Duarte FJ, Santos AG. Directed electrostatic activation in enantioselective organocatalytic cyclopropanation reactions: a computational study. Org Biomol Chem 2016;14:5965-82.
47. Hartikka A, Arvidsson PI. Tetrazolic acid functionalized dihydroindol: rational design of a highly selective cyclopropanation organocatalyst. J Org Chem 2007;72:5874-7.
48. Hartikka A, Ślósarczyk AT, Arvidsson PI. Application of novel sulfonamides in enantioselective organocatalyzed cyclopropanation. Tetrahedron Asymmetr 2007;18:1403-9.
49. Taylor MS, Jacobsen EN. Asymmetric catalysis by chiral hydrogen-bond donors. Angew Chem Int Ed Engl 2006;45:1520-43.
50. Doyle AG, Jacobsen EN. Small-molecule H-bond donors in asymmetric catalysis. Chem Rev 2007;107:5713-43.
51. Alemán J, Parra A, Jiang H, Jørgensen KA. Squaramides: bridging from molecular recognition to bifunctional organocatalysis. Chem Eur J 2011;17:6890-9.
52. Cheng Y, An J, Lu LQ, et al. Asymmetric cyclopropanation of
53. Wang J, Liu X, Dong S, Lin L, Feng X. Asymmetric organocatalytic cyclopropanation of cinnamone derivatives with stabilized sulfonium ylides. J Org Chem 2013;78:6322-7.
54. Lu LQ, Cao YJ, Liu XP, et al. A new entry to cascade organocatalysis: reactions of stable sulfur ylides and nitroolefins sequentially catalyzed by thiourea and DMAP. J Am Chem Soc 2008;130:6946-8.
55. Lu LQ, Li F, An J, Cheng Y, Chen JR, Xiao WJ. Hydrogen-bond-mediated asymmetric cascade reaction of stable sulfur ylides with nitroolefins: scope, application and mechanism. Chem Eur J 2012;18:4073-9.
56. Sekizawa R, Iinuma H, Muraoka Y, et al. Isolation and synthesis of novel farnesyl protein transferase inhibitors, valinoctins A and B, from Streptomyces strain MJ858-NF3. J Nat Prod 1996;59:232-6.
57. Kakeya H, Morishita M, Koshino H, Morita Ti TI, Kobayashi K, Osada H. Cytoxazone: a novel cytokine modulator containing a 2-oxazolidinone ring produced by streptomyces sp. J Org Chem 1999;64:1052-3.
58. Lu LQ, Li F, An J, et al. Construction of fused heterocyclic architectures by formal [4 + 1]/[3 + 2] cycloaddition cascade of sulfur ylides and nitroolefins. Angew Chem Int Ed Engl 2009;48:9542-5.
59. Laurita T, D’orsi R, Chiummiento L, Funicello M, Lupattelli P. Recent advances in synthetic strategies to 2,3-dihydrobenzofurans. Synthesis 2020;52:1451-77.
60. Dapkekar BA, Sreenivasulu C, Kishore DR, Satyanarayana G. Recent advances towards the synthesis of dihydrobenzofurans and dihydroisobenzofurans. Asian J Org Chem 2022;11:e202200012.
61. Lei X, Jiang C, Wen X, Xu Q, Sun H. Formal [4 + 1] cycloaddition of o-quinone methides: facile synthesis of dihydrobenzofurans. RSC Adv 2015;5:14953-7.
62. Yang Q, Xiao W. Catalytic asymmetric synthesis of chiral dihydrobenzofurans through a formal [4 + 1] annulation reaction of sulfur ylides and in situ generated
63. Kakei H, Sone T, Sohtome Y, Matsunaga S, Shibasaki M. Catalytic asymmetric cyclopropanation of enones with dimethyloxosulfonium methylide promoted by a La-Li3-(biphenyldiolate)3 + NaI complex. J Am Chem Soc 2007;129:13410-1.
64. Sone T, Yamaguchi A, Matsunaga S, Shibasaki M. Catalytic asymmetric synthesis of 2,2-disubstituted terminal epoxides
65. Sone T, Lu G, Matsunaga S, Shibasaki M. Catalytic asymmetric synthesis of 2,2-disubstituted oxetanes from ketones by using a one-pot sequential addition of sulfur ylide. Angew Chem Int Ed Engl 2009;48:1677-80.
66. Sone T, Yamaguchi A, Matsunaga S, Shibasaki M. Enantioselective synthesis of 2,2-disubstituted terminal epoxides via catalytic asymmetric Corey-Chaykovsky epoxidation of ketones. Molecules 2012;17:1617-34.
67. Wang L, Cao W, Mei H, Hu L, Feng X. Catalytic asymmetric synthesis of chiral spiro-cyclopropyl oxindoles from 3-alkenyl-oxindoles and sulfoxonium ylides. Adv Synth Catal 2018;360:4089-93.
68. Dias RM, Burtoloso AC. Catalyst-free insertion of sulfoxonium ylides into aryl thiols. A direct preparation of
69. Momo PB, Leveille AN, Farrar EHE, Grayson MN, Mattson AE, Burtoloso ACB. Enantioselective S-H insertion reactions of
70. Eftekhari-Sis B, Zirak M.
71. Najera C, Sansano JM. Catalytic asymmetric synthesis of alpha-amino acids. Chem Rev 2007;107:4584-671.
72. Furniel LG, Echemendía R, Burtoloso ACB. Cooperative copper-squaramide catalysis for the enantioselective N-H insertion reaction with sulfoxonium ylides. Chem Sci 2021;12:7453-9.
73. Guo W, Wang M, Han Z, Huang H, Sun J. Organocatalytic asymmetric synthesis of
74. Ford A, Miel H, Ring A, Slattery CN, Maguire AR, Mckervey MA. Modern organic synthesis with
75. Xia Y, Qiu D, Wang J. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem Rev 2017;117:13810-89.
76. Guo W, Zhou Y, Xie H, et al. Visible-light-induced organocatalytic enantioselective N-H insertion of
77. Zhou Y, Yue X, Jiang F, Sun J, Guo W. Catalytic asymmetric synthesis of
78. Guo W, Jiang F, Li S, Sun J. Organocatalytic asymmetric azidation of sulfoxonium ylides: mild synthesis of enantioenriched
79. Leveille AN, Echemendía R, Mattson AE, Burtoloso ACB. Enantioselective indole insertion reactions of
80. Bisag GD, Pecchini P, Mancinelli M, Fochi M, Bernardi L. Sulfoxonium ylides in aminocatalysis: an enantioselective entry to cyclopropane-fused chromanol structures. Org Lett 2022;24:5468-73.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wang ZH, Sun TJ, Zhang YP, You Y, Zhao JQ, Yin JQ, Yuan WC. Application of sulfonium and sulfoxonium ylides in organocatalyzed asymmetric reaction. Chem Synth 2023;3:12. http://dx.doi.org/10.20517/cs.2023.01
AMA Style
Wang ZH, Sun TJ, Zhang YP, You Y, Zhao JQ, Yin JQ, Yuan WC. Application of sulfonium and sulfoxonium ylides in organocatalyzed asymmetric reaction. Chemical Synthesis. 2023; 3(2): 12. http://dx.doi.org/10.20517/cs.2023.01
Chicago/Turabian Style
Wang, Zhen-Hua, Ting-Jia Sun, Yan-Ping Zhang, Yong You, Jian-Qiang Zhao, Jun-Qing Yin, Wei-Cheng Yuan. 2023. "Application of sulfonium and sulfoxonium ylides in organocatalyzed asymmetric reaction" Chemical Synthesis. 3, no.2: 12. http://dx.doi.org/10.20517/cs.2023.01
ACS Style
Wang, Z.H.; Sun T.J.; Zhang Y.P.; You Y.; Zhao J.Q.; Yin J.Q.; Yuan W.C. Application of sulfonium and sulfoxonium ylides in organocatalyzed asymmetric reaction. Chem. Synth. 2023, 3, 12. http://dx.doi.org/10.20517/cs.2023.01
About This Article
Special Issue
Copyright
Author Biographies







Data & Comments
Data

 Cite This Article 29 clicks
Cite This Article 29 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.