Luminescent alkynylplatinum(II) terpyridine-containing conjugated polymers: synthesis, characterization and photophysical studies
Abstract
A series of alkynylplatinum(II) terpyridine complexes and alkynylplatinum(II) terpyridine-containing conjugated polymers with different polymer backbones has been synthesized, and their spectroscopic properties and Förster resonance energy transfer (FRET) processes has been investigated. The platinum(II)-containing polymers exhibit dual emissive features with emission maxima at ca. 416-465 nm and ca. 671-673 nm, which are assigned to be originated from singlet intraligand (1IL) excited states from the polymer backbone and triplet metal-metal-to-ligand charge transfer (3MMLCT) excited states from the platinum(II) pendants, respectively. The Förster radii (R0) of the platinum(II)-containing conjugated polymers have been determined, and their distinctive thermo-responsive luminescence changes have also been observed. The present work has demonstrated the utilization of “click” reaction for the preparation of platinum(II)-containing conjugated polymers, which show unique photophysical and spectroscopic properties. Through the judicious design, this type of platinum(II)-containing polymer is found to be sensitive to temperature, resulting in ratiometric emission changes. This study has provided valuable insights into the preparation of metal-containing polymeric systems for different applications.
Keywords
INTRODUCTION
Over the past few decades, conjugated polymers, which have been extensively studied with well-known examples such as poly(p-phenylene vinylene) (PPV)[1], polypyrrole (PPy)[2-4], polythiophene (PT)[5] and so on, represent important classes of organic macromolecules, and have found widespread applications in organic photovoltaic devices, light-emitting diodes, sensing materials, and others[6-11]. The prominence of conjugated polymers can be attributed to their unique properties of high planarity and extended π-electron delocalization, empowering them with rich photophysical and electrochemical functionalities for specific applications[6-11]. After the success in designing and synthesizing different kinds of conjugated polymers, attempts have been made to integrate conjugated polymers with transition metals, namely metallo-conjugated polymers, with a view to not only improving the physical properties of the parent organic polymers such as mechanical strength, thermal stability and carrier mobility but also enriching their photophysical properties such as harvesting energy from the triplet excited state and extending the absorption spectrum to the red or near-infrared (NIR) region[12-19]. Earlier examples include ruthenium(II)-containing conjugated polymers with poly(bpy-co-benzobisoxazole)s or poly(bpy-co-benzobisthiazole)s as the polymer backbones[20] and iridium(III)-containing conjugated polymers with polyfluorene as the polymer backbone and carbazole unit as the pendant[21]. Unlike most other commonly studied transition metal centers, including ruthenium(II), rhodium(III) and iridium(III), d8 platinum(II) center favors coordination of a square-planar geometry, and their complexes, especially those bearing conjugated aromatic ligands capable of exhibiting π-π interactions, are well-known for their ability to self-assemble[22-26], forming aggregates[27-31] and providing remarkable photophysical properties associated with Pt···Pt and π-π interactions[32-35]. In light of their supramolecular assembly capability, it is envisaged that the introduction of platinum centers into conjugated polymers may provide an opportunity to further modulate the photophysical and morphological properties of the resulting metal–organic hybrid materials[36-38]. Although there were examples of platinum(II)-containing conjugated polymers such as platinum(II) polyynes[39-46] and cyclometalating bidentate ligand-containing platinum(II)-based conjugated polymers[47-49], none of these examples demonstrates supramolecular assembly properties or utilizes the system of tridentate N-donor ligands. In this work, a series of alkynylplatinum(II) terpyridine complexes (1 and 2) and alkynylplatinum(II) terpyridine-containing conjugated polymers with different polymer backbones (3-5)
EXPERIMENTAL
Syntheses of conjugated polymers and complexes 1-5
The synthetic routes for platinum(II) precursor and reference complex are depicted in
The synthetic routes for the conjugated polymers are depicted in Supplementary Scheme 2. Detailed syntheses of the bromo-containing conjugated polymers, poly[fluorene(C6H12Br)2-co-fluorene(C6H13)2]
The synthetic routes for the platinum(II)-containing conjugated polymers are depicted in Supplementary Scheme 3. The platinum(II)-containing conjugated polymers 3-5 were also obtained through copper(I)-catalyzed alkyne-azide cycloaddition of the corresponding azido-containing conjugated polymers and 1 in THF-DMF mixture in the presence of ammonium triflate (pp S15-S17, Supplementary Materials). The products were purified by precipitation in deionized water containing ammonium triflate. The platinum(II)-containing conjugated polymers were found to have fair solubility in acetonitrile, DMF and DMSO. Their limited solubility in methanol and THF has facilitated the purification by washing the precipitate with methanol and THF to further remove any unreacted starting materials.
Characterization
All the newly synthesized platinum(II) complexes 1 and 2 and platinum(II)-containing polymers 3-5 have been characterized by 1H NMR and IR spectroscopy. In addition, 1 and 2 were also confirmed by positive-ion FAB mass spectrometry and showed satisfactory results in the elemental analyses. 3-5 were also confirmed by GPC analysis using DMF with 0.1 M KPF6 as eluent. Representative GPC data of 5 is provided in Supplementary Figure 1.
From the IR measurements of 3-5 [Supplementary Figures 2-4], the disappearance of the strong absorption of the N=N=N stretch of the azide precursor at ca. 2095 cm-1, the appearance of weak absorption of the C≡C stretch at 2110 cm-1 and strong absorption of the triflate counter-ion at ca. 1155 and 1030 cm-1 indicated the successful incorporation of the platinum(II) complexes onto the polymer via “click” reaction.
RESULTS AND DISCUSSION
The polymers, PF-Br, PFP-Br and PFT-Br, are soluble in dichloromethane and give high-energy absorption bands with a peak maxima at ca. 375-398 nm [Supplementary Figure 5 and Supplementary Table 1], which are assigned as the π→π* transitions along the polymer backbone, while these polymers show strong vibronic-structured emissions with peak maxima at ca. 410-462 nm upon photoexcitation [Supplementary Figures 6-9 and Supplementary Table 2], which are assigned as the singlet [π→π*] fluorescence of the conjugated polymer backbone.
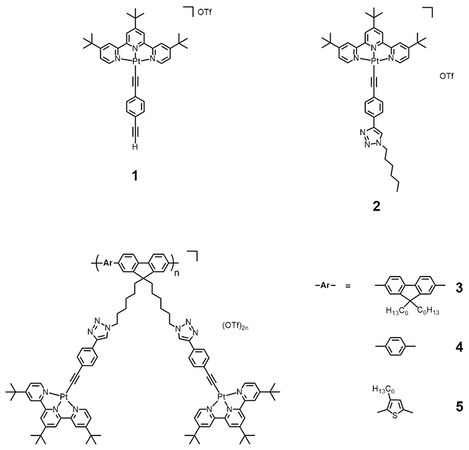
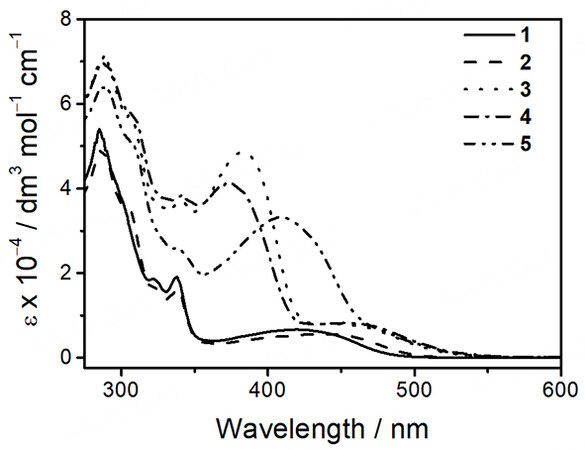
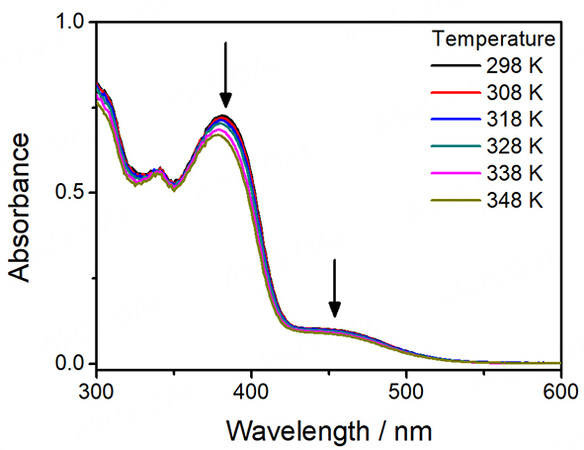
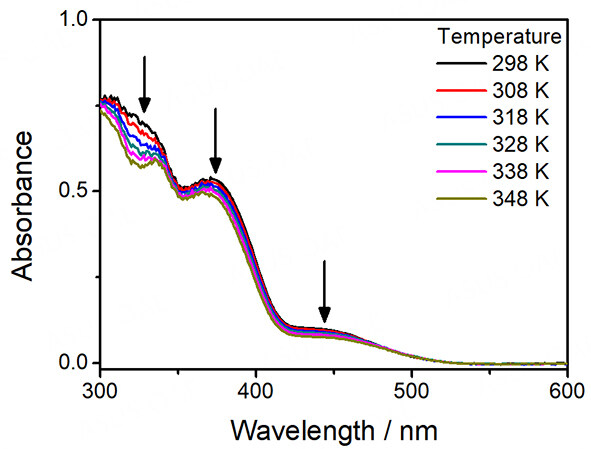
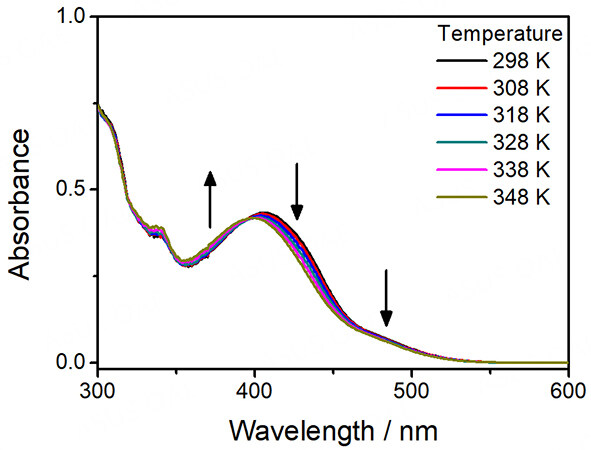
For complexes 1-5, they all give pale yellow solutions in acetonitrile. Their corresponding UV-vis absorption data and spectra in acetonitrile at 298 K are depicted in Table 1 and Figure 1, respectively. All the complexes exhibit intense absorption bands at ca. 285-341 nm with molar extinction coefficients in the order of 104 dm3 mol-1 cm-1 and less intense low-energy absorption bands at ca. 420-466 nm with molar extinction coefficients in the order of 103 dm3 mol-1 cm-1. The higher-energy bands are ascribed to intraligand (IL) [π→π*] transitions of alkynyl and terpyridine ligands, while the lower-energy bands are assigned as an admixture of metal-to-ligand charge transfer (MLCT) [dπ(Pt)→π*(tpy)] and ligand-to-ligand charge transfer (LLCT) [π(alkynyl)→π*(tpy)] transitions. For the platinum(II)-containing conjugated polymers 3-5, intense absorption bands at ca. 374-409 nm have been observed. With reference to the previous studies on the conjugated polymers[51-54] and the UV-vis absorption studies of the corresponding organic polymers [Supplementary Figure 5], these absorptions are tentatively assigned as the IL [π→π*] transitions of the polymer backbones. Interestingly, the lower-energy bands of 3-5 are extended to longer wavelengths when compared to the reference complex 2. Since the molecular structures of the platinum(II) pendants in 3-5 are the same as that in 2, the further red-shifted absorption tails suggest the existence of metal-metal-to-ligand charge transfer (MMLCT) character. As such, concentration-dependent UV-vis absorption studies have been performed. Based on the spectra [Supplementary Figures 10-14], the precursor platinum(II) complexes 1 and 2 and the platinum(II)-containing conjugated polymers 3-5 show good agreement with Beer’s Law, suggesting that there are no significant intermolecular self-assembly properties of 1-5 upon increasing the concentration. However, the intramolecular self-assembly mode of having two platinum(II) pendants in each repeating unit, which are stabilized by the presence of intramolecular Pt···Pt and π-π interactions, may explain the presence of low-energy MMLCT bands for the platinum(II)-containing conjugated polymers 3-5. In this regard, temperature-dependent UV-vis absorption experiments for 3-5 have been carried out. From the spectra [Figures 2-4], the low-energy band at ca. 450 nm shows a drop in absorbance accompanied by a blue shift of the high-energy band at ca. 400 nm upon increasing temperature, suggesting the occurrence of deaggregation process of both the platinum(II) terpyridine moieties and the polymer backbones, which corroborates with the disruption of intramolecular Pt···Pt and π-π interactions at high temperatures.
Figure 2. UV-Vis absorption spectral changes of [PF-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (3) in acetonitrile with increasing temperature.
Figure 3. UV-Vis absorption spectral changes of [PFP-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (4) in acetonitrile with increasing temperature.
Figure 4. UV-Vis absorption spectral changes of [PFT-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (5) in acetonitrile with increasing temperature.
UV-Vis absorption data for 1-5 at 298 K
| Complex | Medium | Absorption λ/nm (ε/dm3 mol-1 cm-1) |
| [Pt(tBu3tpy)(C≡CC6H4C≡CH)]OTf (1) | CH3CN | 285 (52,030), 322 (18,620), 338 (19,130), 420 (6,660) |
| [Pt(tBu3tpy)(C≡CC6H4C2HN3C6H13)]OTf (2) | CH3CN | 287 (49,290), 305 sh (35,150), 324 sh (16,330), 338 (15,980), 401 sh (15,980), 434 (5,560) |
| [PF-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n | CH3CN | 288 (71,290), 308 sh (55,900), 341 (37,020), 383 (48,560), 445 sh (8,010) |
| [PFP-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (4)a | CH3CN | 287 (69,780), 308 sh (57,730), 341 (38,350), 374 (41,140), 451 sh (8,190) |
| [PFT-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (5)a | CH3CN | 289 (64,250), 308 sh (50,700), 340 sh (25,560), 409 (33,250), 466 sh (8,320) |
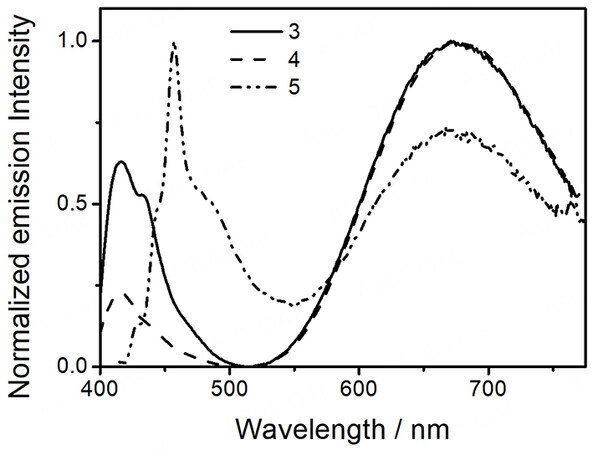
Complexes 1 and 2 are found to give phosphorescence in degassed solutions, while dual-emissive behaviors have been observed for the platinum(II)-containing conjugated polymers 3-5 in degassed solutions upon excitation. The luminescence data of all complexes have been summarized in Table 2. Upon photoexcitation at λ > 350 nm, 1 and 2 show Gaussian-shape emission bands centered at 596 nm and 630 nm in degassed acetonitrile [Supplementary Figure 15]. The large Stokes shifts and the long emission lifetimes in the microsecond regime indicate that these emissions are originated from a triplet parentage. Together with the relatively short photoluminescence lifetimes in the range of 1 ms or lower, these emission bands are assigned to be originated from admixtures of
Emission data for 1-5
| Complex | Medium (T/K) | λem/nm (τo/μs) | Φluma |
| [Pt(tBu3tpy)(C≡CC6H4C≡CH)]OTf (1) | CH3CN (298) | 596 (1.09) | 5.0 × 10-2b |
| [Pt(tBu3tpy)(C≡CC6H4-C2HN3C6H13)]OTf (2) | CH3CN (298) | 630 (0.14) | 8.5 × 10-3b |
| [PF-{N3C2H-C6H4C≡C-Pt(tBu3tpy)}2](OTf)2n (3) | CH3CN (298) | 416c (< 0.1), 671 (0.14) | 1.2 × 10-3d 2.6 × 10-3b |
| [PFP-{N3C2H-C6H4C≡C-Pt(tBu3tpy)}2](OTf)2n (4) | CH3CN (298) | 417c (< 0.1), 673 (0.70) | 9.0 × 10-4d 1.4 × 10-2b |
| [PFT-{N3C2H-C6H4C≡C-Pt(tBu3tpy)}2](OTf)2n (5) | CH3CN (298) | 465c (< 0.1), 673 (0.66) | 8.4 × 10-4d 1.7 × 10-3b |
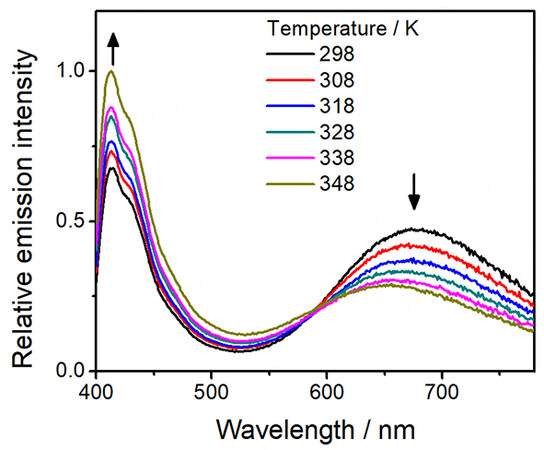
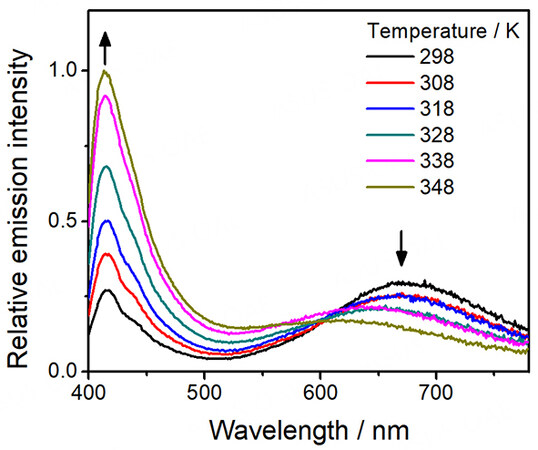
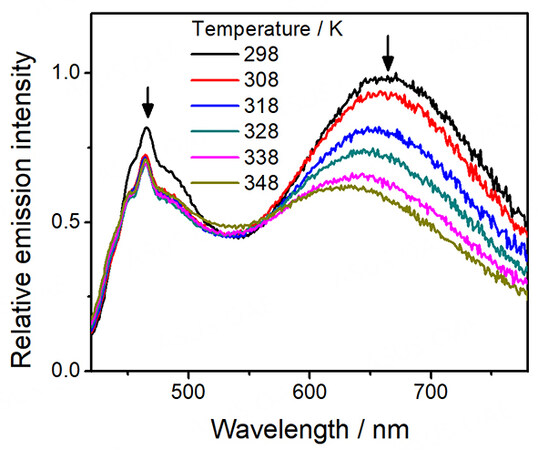
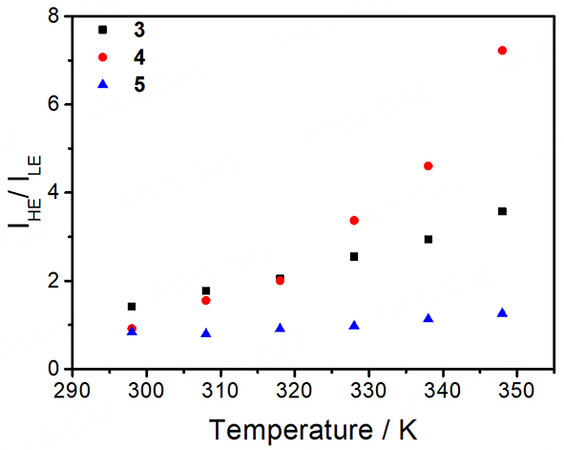
On the other hand, distinctive thermo-responsive emission changes have also been observed for the platinum(II)-containing conjugated polymers 3-5. Upon increasing the temperature of the solution of 3, the high-energy emission from the polymer backbone is found to increase in intensity [Figure 6]. The reason behind this can be attributed to the decrease in FRET efficiency from the polymer backbone to the platinum(II) moieties. From the variable-temperature UV-vis absorption spectral traces of 3 [Figure 2], there is a decrease in absorbance of the MMLCT band upon increasing temperature, leading to a decrease in the spectral overlap and the enhanced recovery of the polymer fluorescence [Figure 6]. Moreover, 4 is found to exhibit the largest recovery of the high-energy emission when compared to others upon increasing temperature, as shown in Figure 7. Since 4 bears the least number of alkyl chains in each repeating unit, it is believed that the energy would be less effectively dissipated through non-radiative decay pathways. As a result, the FRET process dominates in 4, resulting in the greatest recovery of the polymer backbone emission. Furthermore, both emission bands of 5 are found to be diminished with increasing temperature [Figure 8], which can be attributed to the more dominating non-radiative process when compared to the recovery of the fluorescence of the polymer backbone. The corresponding ratiometric emission intensity plots of 3-5 have been depicted in Figure 9.
Figure 6. Emission spectra of [PF-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (3) in acetonitrile with increasing temperature.
Figure 7. Emission spectra of [PFP-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (4) in acetonitrile with increasing temperature.
Figure 8. Emission spectra of [PFT-{N3C2H-C6H4C≡CPt(tBu3tpy)}2](OTf)2n (5) in acetonitrile with increasing temperature.
Figure 9. Ratiometric emission intensity plots of the high-energy (HE) and low-energy (LE) bands of 3-5 in acetonitrile with increasing temperature. IHE/ILE of 3 = I413nm/I673nm; IHE/ILE of 4 = I413nm/I673nm; IHE/ILE of 5 = I465nm/I673nm.
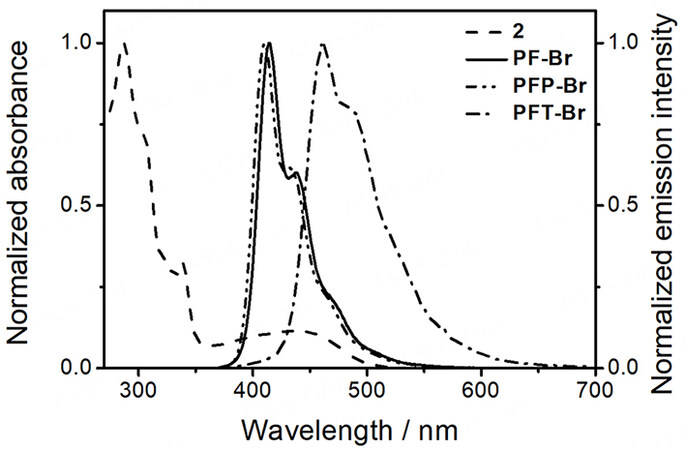
Due to the good spectral overlap between the absorption spectrum of the reference complex 2 and the emission spectra of the conjugated polymers (PF-Br, PFP-Br and PFT-Br) [Figure 10], it is believed that the intramolecular FRET process from the polymer backbone to the platinum(II) pendant would likely occur upon photoexcitation. Although the emissions from the conjugated polymer backbones could still be observed for 3-5, they are already effectively quenched when compared to their corresponding organic polymers (Φlum of PF-Br, PFP-Br and PFT-Br = 0.45-0.92; Φlum of 3-5 = 8.4 × 10-4-1.2 × 10-3). It is worth noting that different extents of quenching efficiencies have been observed for 3-5. For example, the emission from the polymer backbone of 5 is found to be less effectively quenched when compared to that of 3 and 4. The related parameters have been obtained and are summarized in Table 3. Since the platinum(II)-containing conjugated polymers 3-5 share similar molecular structures except for the polymer backbone, it is believed that the values of the relative orientation of the transition dipoles of the chromophores (κ) and the distance between the donor and the acceptor (r) should be almost the same. Therefore, the FRET efficiency is mainly governed by the emission quantum yield of the donor (ΦD) and the spectral overlap integral of the absorption spectrum of the acceptor and emission spectrum of the donor (J(λ)), which are related to the Förster radius (R0). It is found that the calculated R0 value of 5 is the lowest, indicating that the FRET in 5 should be the least efficient, as reflected by the smallest decrease in emission quantum yield of the polymer backbone.
Figure 10. Normalized UV-vis absorption of [Pt(tBu3tpy)(C≡CC6H4-C2HN3C6H13)]OTf (2) and emission spectra of PF-Br, PFP-Br and PFT-Br showing the spectral overlap between the emission spectra of the polymer energy donors and the UV-vis absorption spectrum of the platinum(II) complex 2 energy acceptor.
Parameters obtained from the equation determining the Förster radius, R0a of 3-5
| Acceptor | Donor | ΦDb,c | R0/nm | Pt(II)-Polymer | Φlumb,c,d | Φlum/ΦD |
| 2 | PF-Br | 0.92 | 4.9 | 3 | 1.2 × 10-3 | 1.30 × 10-3 |
| 2 | PFP-Br | 0.90 | 4.9 | 4 | 9.0 × 10-4 | 1.00 × 10-3 |
| 2 | PFT-Br | 0.45 | 4.5 | 5 | 8.4 × 10-4 | 1.87 × 10-3 |
CONCLUSION
Alkynylplatinum(II) terpyridine complexes (1 and 2) and alkynylplatinum(II) terpyridine-containing conjugated polymers with different polymer backbones (3-5) have been prepared, and their spectroscopic properties as well as FRET processes have been studied. The platinum(II)-containing polymers 3-5 are found to exhibit dual emissive features, in which the two emission bands correspond to 1IL fluorescence from the polymer backbones and 3MMLCT emissions from the platinum(II) pendants. Such unique luminescence behavior is attributed to the intramolecular Pt···Pt and/or π-π interactions between the platinum(II) pendants in the polymer molecules. The FRET processes between the conjugated polymer backbones and platinum(II) pendants have been studied systemically. It is found that 5 has the lowest Förster radii (R0) among others, probably due to the lowest emission quantum yield of poly(fluorene-co-thiophene). Distinctive thermo-responsive ratiometric emission changes have been observed for 3 and 4, in which an increase in intensity of the high-energy 1IL emission originated from the polymer backbones and a decrease in intensity of the low-energy 3MMLCT emission are found upon heating. The present work has demonstrated the utilization of “click” reaction for the convenient preparation of platinum(II)-containing conjugated polymers, which show unique photophysical and spectroscopic properties. Through the judicious design, ratiometric emission changes upon varying temperatures have been realized in this class of platinum(II)-containing polymers. This study may provide valuable insights into the preparation of metal-containing polymeric systems for different applications, such as thermochromic materials. Owing to the ease of structural modifications, various kinds of polymeric materials could be potentially fabricated, which could serve as thermochromic sensors for monitoring temperature in real time.
DECLARATIONS
Authors’ contributionsConducted the synthesis, characterization and photophysical measurements, analyzed the data and prepared the manuscript: Cheng HK
Initiated and designed the research, analyzed the data and prepared the manuscript: Yam VWW
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the Collaborative Research Fund (CRF) (C7075-21G) and the General Research Fund (GRF) from the Research Grants Council of the Hong Kong Special Administrative Region, People’s Republic of China (HKU17303421), and the CAS-Croucher Funding Scheme for Joint Laboratory on Molecular Functional Materials for Electronics, Switching and Sensing. H.-K.C. acknowledges the receipt of a Postgraduate Studentship.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Burroughes JH, Bradley DDC, Brown AR, et al. Light-emitting diodes based on conjugated polymers. Nature 1990;347:539-41.
2. Mcneill R, Siudak R, Wardlaw J, Weiss D. Electronic conduction in polymers. I. The chemical structure of polypyrrole. Aust J Chem 1963;16:1056-75.
3. Bolto B, Weiss D. Electronic conduction in polymers. II. The electrochemical reduction of polypyrrole at controlled potential. Aust J Chem 1963;16:1076-89.
4. Bolto B, Mcneill R, Weiss D. Electronic conduction in polymers. III. Electronic properties of polypyrrole. Aust J Chem 1963;16:1090-103.
6. Sirringhaus H, Tessler N, Friend RH. Integrated optoelectronic devices based on conjugated polymers. Science 1998;280:1741-4.
7. Ago H, Petritsch K, Shaffer MSP, Windle AH, Friend RH. Composites of carbon nanotubes and conjugated polymers for photovoltaic devices. Adv Mater 1999;11:1281-5.
8. Friend RH, Gymer RW, Holmes AB, et al. Electroluminescence in conjugated polymers. Nature 1999;397:121-8.
9. Sirringhaus H, Brown PJ, Friend RH, et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999;401:685-8.
10. Mcgehee MD, Heeger AJ. Semiconducting (conjugated) polymers as materials for solid-state lasers. Adv Mater 2000;12:1655-68.
11. Thomas SW 3rd, Joly GD, Swager TM. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 2007;107:1339-86.
12. Ho CL, Wong WY. Metal-containing polymers: facile tuning of photophysical traits and emerging applications in organic electronics and photonics. Coord Chem Rev 2011;255:2469-502.
13. Gracia R, Mecerreyes D. Polymers with redox properties: materials for batteries, biosensors and more. Polym Chem 2013;4:2206.
14. Liu S, Zhang K, Lu J, et al. High-efficiency polymer solar cells via the incorporation of an amino-functionalized conjugated metallopolymer as a cathode interlayer. J Am Chem Soc 2013;135:15326-9.
15. Du M, Li C, Liu C, Fang S. Design and construction of coordination polymers with mixed-ligand synthetic strategy. Coord Chem Rev 2013;257:1282-305.
16. Xu H, Chen R, Sun Q, et al. Recent progress in metal-organic complexes for optoelectronic applications. Chem Soc Rev 2014;43:3259-302.
17. Winter A, Schubert US. Synthesis and characterization of metallo-supramolecular polymers. Chem Soc Rev 2016;45:5311-57.
18. Ho CL, Yu ZQ, Wong WY. Multifunctional polymetallaynes: properties, functions and applications. Chem Soc Rev 2016;45:5264-95.
19. Götz S, Zechel S, Hager MD, Newkome GR, Schubert US. Versatile applications of metallopolymers. Prog Polym Sci 2021;119:101428.
20. Yu SC, Gong X, Chan WK. Synthesis and characterization of poly(benzobisoxazole)s and poly(benzobisthiazole)s with 2,2’-bipyridyl units in the backbone. Macromolecules 1998;31:5639-46.
21. Chen X, Liao JL, Liang Y, Ahmed MO, Tseng HE, Chen SA. High-efficiency red-light emission from polyfluorenes grafted with cyclometalated iridium complexes and charge transport moiety. J Am Chem Soc 2003;125:636-7.
22. Yam VWW, Wong KMC, Zhu N. Solvent-induced aggregation through metal···metal/π···π interactions: large solvatochromism of luminescent organoplatinum(II) terpyridyl complexes. J Am Chem Soc 2002;124:6506-7.
23. Yam VWW, Chan KHY, Wong KMC, Zhu N. Luminescent platinum(II) terpyridyl complexes: effect of counter ions on solvent-induced aggregation and color changes. Chem Eur J 2005;11:4535-43.
24. Yu C, Wong KMC, Chan KHY, Yam VWW. Polymer-induced self-assembly of alkynylplatinum(II) terpyridyl complexes by metal···metal/π···π interactions. Angew Chem Int Ed 2005;44:791-4.
25. Yu C, Chan KHY, Wong KMC, Yam VWW. Single-stranded nucleic acid-induced helical self-assembly of alkynylplatinum(II) terpyridyl complexes. Proc Natl Acad Sci USA 2006;103:19652-7.
26. Leung SYL, Lam WH, Yam VWW. Dynamic scaffold of chiral binaphthol derivatives with the alkynylplatinum(II) terpyridine moiety. Proc Natl Acad Sci USA 2013;110:7986-91.
27. Wong KMC, Yam VWW. Luminescence platinum(II) terpyridyl complexes - From fundamental studies to sensory functions. Coord Chem Rev 2007;251:2477-88.
28. Po C, Tam AYY, Wong KMC, Yam VWW. Supramolecular self-assembly of amphiphilic anionic platinum(II) complexes: a correlation between spectroscopic and morphological properties. J Am Chem Soc 2011;133:12136-43.
29. Wong KMC, Yam VWW. Self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes: modulation of photophysical properties through aggregation behavior. Acc Chem Res 2011;44:424-34.
30. Cheung ASH, Leung SYL, Hau FKW, Yam VWW. Supramolecular self-assembly of amphiphilic alkynylplatinum(II) 2,6-bis(N-alkylbenzimidazol-2’-yl)pyridine complexes. Chem Res Chin Univ 2021;37:1079-84.
31. Zheng X, Chan MHY, Chan AKW, et al. Elucidation of the key role of Pt···Pt interactions in the directional self-assembly of platinum(II) complexes. Proc Natl Acad Sci USA 2022;119:e2116543119.
32. Tam AYY, Wong KMC, Yam VWW. Unusual luminescence enhancement of metallogels of alkynylplatinum(II) 2,6-bis(N-alkylbenzimidazol-2’-yl)pyridine complexes upon a gel-to-sol phase transition at elevated temperatures. J Am Chem Soc 2009;131:6253-60.
33. Yam VWW, Au VKM, Leung SYL. Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem Rev 2015;115:7589-728.
34. Yam VWW, Chan AKW, Hong EYH. Charge-transfer processes in metal complexes enable luminescence and memory functions. Nat Rev Chem 2020;4:528-41.
35. Chan MHY, Yam VWW. Toward the design and construction of supramolecular functional molecular materials based on metal-metal interactions. J Am Chem Soc 2022;144:22805-25.
36. Chan K, Chung CYS, Yam VWW. Conjugated polyelectrolyte-induced self-assembly of alkynylplatinum(II) 2,6-bis(benzimidazol-2’-yl)pyridine complexes. Chem Eur J 2015;21:16434-47.
37. Chan K, Chung CYS, Yam VWW. Parallel folding topology-selective label-free detection and monitoring of conformational and topological changes of different G-quadruplex DNAs by emission spectral changes via FRET of mPPE-Ala-Pt(II) complex ensemble. Chem Sci 2016;7:2842-55.
38. Chan CWT, Chan K, Yam VWW. Induced self-assembly and disassembly of alkynylplatinum(II) 2,6-bis(benzimidazol-2’-yl)pyridine complexes with charge reversal properties: “proof-of-principle” demonstration of ratiometric förster resonance energy transfer sensing of pH. ACS Appl Mater Interfaces 2022;Online ahead of print.
39. Sonogashira K, Takahashi S, Hagihara N. A new extended chain polymer, poly[trans-bis(tri-
40. Takahashi S, Kariya M, Yatake T, Sonogashira K, Hagihara N. Studies of poly-yne polymers containing transition metals in the main chain. 2. Synthesis of poly[trans-bis(tri-
41. Beljonne D, Wittmann HF, Köhler A, et al. Spatial extent of the singlet and triplet excitons in transition metal-containing poly-ynes. J Chem Phys 1996;105:3868-77.
42. Younus M, Köhler A, Cron S, et al. Synthesis, electrochemistry, and spectroscopy of blue platinum(II) polyynes and diynes. Angew Chem Int Ed Engl 1998;37:3036-9.
43. Chawdhury N, Köhler A, Friend RH, et al. Evolution of lowest singlet and triplet excited states with number of thienyl rings in platinum poly-ynes. J Chem Phys 1999;110:4963-70.
44. Rogers JE, Cooper TM, Fleitz PA, Glass DJ, Mclean DG. Photophysical characterization of a series of platinum(II)-containing phenyl−ethynyl oligomers. J Phys Chem A 2002;106:10108-15.
45. Liu Y, Jiang S, Glusac K, Powell DH, Anderson DF, Schanze KS. Photophysics of monodisperse platinum-acetylide oligomers: delocalization in the singlet and triplet excited states. J Am Chem Soc 2002;124:12412-3.
46. Schanze KS, Silverman EE, Zhao X. Intrachain triplet energy transfer in platinum-acetylide copolymers. J Phys Chem B 2005;109:18451-9.
47. Clem TA, Kavulak DFJ, Westling EJ, Fréchet JMJ. Cyclometalated platinum polymers: synthesis, photophysical properties, and photovoltaic performance. Chem Mater 2010;22:1977-87.
48. Thomas III SW, Yagi S, Swager TM. Towards chemosensing phosphorescent conjugated polymers: cyclometalated platinum(II) poly(phenylene)s. J Mater Chem 2005;15:2829.
49. Wang P, Liu S, Lin Z, et al. Design and synthesis of conjugated polymers containing Pt(II) complexes in the side-chain and their application in polymer memory devices. J Mater Chem 2012;22:9576.
50. Lu W, Law YC, Han J, et al. A dicationic organoplatinum(II) complex containing a bridging 2,5-bis-(4-ethynylphenyl)-[1,3,4]oxadiazole ligand behaves as a phosphorescent gelator for organic solvents. Chem Asian J 2008;3:59-69.
51. Liu B, Yu W, Lai Y, Huang W. Blue-light-emitting fluorene-based polymers with tunable electronic properties. Chem Mater 2001;13:1984-91.
52. Vamvounis G, Schulz GL, Holdcroft S. Enhanced blue-violet emission from poly(fluorene-
53. Grell M, Bradley DDC, Ungar G, Hill J, Whitehead KS. Interplay of physical structure and photophysics for a liquid crystalline polyfluorene. Macromolecules 1999;32:5810-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cheng HK, Yam VW. Luminescent alkynylplatinum(II) terpyridine-containing conjugated polymers: synthesis, characterization and photophysical studies. Chem Synth 2023;3:13. http://dx.doi.org/10.20517/cs.2022.43
AMA Style
Cheng HK, Yam VW. Luminescent alkynylplatinum(II) terpyridine-containing conjugated polymers: synthesis, characterization and photophysical studies. Chemical Synthesis. 2023; 3(2): 13. http://dx.doi.org/10.20517/cs.2022.43
Chicago/Turabian Style
Cheng, Heung-Kiu, Vivian Wing-Wah Yam. 2023. "Luminescent alkynylplatinum(II) terpyridine-containing conjugated polymers: synthesis, characterization and photophysical studies" Chemical Synthesis. 3, no.2: 13. http://dx.doi.org/10.20517/cs.2022.43
ACS Style
Cheng, H.K.; Yam V.W. Luminescent alkynylplatinum(II) terpyridine-containing conjugated polymers: synthesis, characterization and photophysical studies. Chem. Synth. 2023, 3, 13. http://dx.doi.org/10.20517/cs.2022.43
About This Article
Special Issue
Copyright
Author Biographies


Data & Comments
Data
 Cite This Article 37 clicks
Cite This Article 37 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.