Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles
Abstract
Quinidine-catalyzed regio- and enantioselective formal [4 + 2]-cycloadditions of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates with N-tosyl-2-methylenebut-3-enoates and 2-methylene-3-oxoalkanoates have been developed for the first time. The reaction features the in situ formation of chiral nitrogen-containing dipolar intermediates, a ring-opening/Michael addition/annulation cascade reaction, and works well over a broad substrate scope to furnish the tetrahydroquinolines in high yields with high asymmetric induction under mild conditions.
Keywords
INTRODUCTION
The enantioenriched tetrahydroquinoline subunit is widely present in compounds with a wide range of biological activities [Scheme 1A][1]. Moreover, chiral 1,2,3,4-tetrahydroquinoline phosphoramidites have also been proven to be promising ligands in Ir-catalyzed asymmetric reactions[2]. Accordingly, the development of new methodologies for the catalyzed asymmetric synthesis of these significant frameworks continues to be a very active field of research[3-7]. Particularly, metal-catalyzed decarboxylative transformations of vinyl benzoxazinones have been identified as a powerful and versatile tool for the asymmetric synthesis of chiral 1,2,3,4-tetrahydroquinolines, which featured a chiral metal-stabilized 1,4-zwitterionic intermediate [Scheme 1B][8].
Notably, organocatalytic asymmetric annulations have emerged as a key platform for the asymmetric construction of functionalized carbo- and heterocycles[9-12], but the organocatalytic asymmetric reactions of benzoxazinones for the construction of chiral tetrahydroquinoline motif remained a challenge [Scheme 1C].
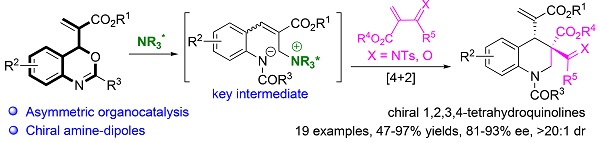
An important breakthrough in the field of the organocatalytic asymmetric reactions of benzoxazinones was reported by Lu et al. in 2018[13]. They replaced the vinyl group of vinyl benzoxazinones with an alkynyl residue, enabling the formation of chiral N-heterocyclic carbene (NHC)-azolium enolate intermediate followed by [4 + 2]-annulation to furnish the chiral 3,4-dihydroquinolin-2(1H)-ones [Scheme 2A]. Based on our work on organocatalytic asymmetric reactions of Morita-Baylis-Hillman (MBH) adducts[14], we modified the structure of vinyl benzoxazinones and successfully developed 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates as new synthons to realize the chiral phosphine-catalyzed enantioselective formal [4 + 2]-cycloadditions via chiral phosphine-dipole intermediate [Scheme 2B][15]. To develop new catalytic systems and explore reactions of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates, we here reported a chiral amine-catalyzed regio- and enantioselective formal [4 + 2]-annulation of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates with α, β-unsaturated carbonyl derivatives for the asymmetric construction of enantioenriched 1,2,3,4-tetrahydroquinolines [Scheme 2C]. Importantly, the strategy represents the first time the chiral amine catalyzed the formal [4 + 2]-annulation of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates via a chiral amine-dipole intermediate.
EXPERIMENTAL
To a solution of CH2Cl2 (0.1 mL) were added 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates 1 (0.1 mmol), α,β-unsaturated carbonyl derivatives 2 or 4 (0.2 mmol) and quinidine C5 (0.01 mmol, catalyst). The mixture was stirred at 35 °C for 96 h. After removing the solvent under vacuum, the residue was purified by flash chromatography (petroleum ether/ethyl acetate = 4/1, v/v) to afford the desired products 3 or 5.
RESULTS AND DISCUSSION
At the outset, we wanted to develop an organocatalytic [4 + 4]-annulation of 2-(4H-benzo[d][1,3]oxazin-
Condition optimization
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.in.T1.001.jpg) | ||||||
| Entrya | Catalyst | Solvent | Temp. | Time (h) | Yield (%)b | ee (%)c |
| 1 | C1 | CH2Cl2 | rt | 24 | 60 | 18 |
| 2 | C2 | CH2Cl2 | rt | 24 | 47 | 40 |
| 3 | C3 | CH2Cl2 | rt | 24 | 14 | 5 |
| 4 | C4 | CH2Cl2 | rt | 24 | 13 | 91 |
| 5 | C5 | CH2Cl2 | rt | 24 | 30 | 90 |
| 6 | C6 | CH2Cl2 | rt | 24 | 9 | 85 |
| 7 | C7 | CH2Cl2 | rt | 24 | 13 | 89 |
| 8 | C5 | CHCl3 | rt | 36 | 15 | 89 |
| 9 | C5 | ClCH2CH2Cl | rt | 36 | trace | - |
| 10 | C5 | EtOAc | rt | 36 | trace | - |
| 11 | C5 | toluene | rt | 36 | trace | - |
| 12 | C5 | THF | rt | 36 | trace | - |
| 13 | C5 | MeCN | rt | 36 | 20 | 87 |
| 14 | C5 | CH2Cl2 (0.4 mL) | rt | 36 | 35 | 89 |
| 15 | C5 | CH2Cl2 (0.3 mL) | rt | 36 | 38 | 89 |
| 16 | C5 | CH2Cl2 (0.2 mL) | rt | 36 | 44 | 89 |
| 17 | C5 | CH2Cl2 (0.1 mL) | rt | 36 | 52 | 89 |
| 18 | C5 | CH2Cl2 (0.05 mL) | rt | 36 | 43 | 89 |
| 19 | C5 | CH2Cl2 (0.1 mL) | rt | 72 | 73 | 89 |
| 20 | C5 | CH2Cl2 (0.1 mL) | rt | 96 | 78 | 89 |
| 21 | C5 | CH2Cl2 (0.1 mL) | rt | 120 | 81 | 89 |
| 22 | C5 | CH2Cl2 (0.1 mL) | rt | 144 | 84 | 89 |
| 23d | C5 | CH2Cl2 (0.1 mL) | rt | 96 | 80 | 89 |
| 24e | C5 | CH2Cl2 (0.1 mL) | rt | 96 | 87 | 89 |
| 25e | C5 | CH2Cl2 (0.1 mL) | 35 °C | 96 | 90 | 89 |
| 26e | C5 | CH2Cl2 (0.1 mL) | 40 °C | 96 | 76 | 89 |
| 27f | C5 | CH2Cl2 (0.1 mL) | 35 °C | 96 | 95 | 89 |
With the optimal conditions in hand, we then investigated the substrate scope. The scope of 2-(4H-benzo
Scheme 3. Substrate scope of the reaction between benzoxazines 1 and N-tosyl-2-methylenebut-3-enoates 2. A mixture of 1 (0.1 mmol), 2 (0.2 mmol), and C5 (10 mol%) in CH2Cl2 (0.1 mL) was stirred at 35 °C for 96 h. All dr > 20:1, determined by 1H NMR. Products 3 were obtained in isolated yield. The enantiomeric excess (ee) was determined by chiral-HPLC analysis.
To further explore the scope of C5-mediated asymmetric formal [4 + 2]-annulation of 2-(4H-benzo
Scheme 4. Substrate scope of the reaction between benzoxazines 1 and 2-methylene-3-oxoalkanoates 4. A mixture of 1 (0.1 mmol), 4 (0.2 mmol), and C5 (10 mol%) in CH2Cl2 (0.1 mL) was stirred at 35 °C for 96 h. All dr > 20:1, determined by 1H NMR. Products 5 were obtained in isolated yield. The enantiomeric excess (ee) was determined by chiral-HPLC analysis.
To demonstrate the utility of this methodology, more investigations were carried out. Pleasingly, the C5-catalyzed reaction was easily scaled up [Scheme 5A]. Under the standard conditions, 5.0 mmol of 2-(2-phenyl-4H-benzo[d][1,3]oxazin-4-yl)acrylate 1a reacted smoothly with 10.0 mmol of 2-[phenyl(tosylimino)methyl]acrylate 2a, affording 2.4 g (75% yield) of 3aa with 89% ee and > 20:1 dr. Treated with N-hydroxybenzimidoyl chloride/Et3N, product 6aa was obtained in 96% yield with 79% ee and > 20:1 dr [Scheme 5B]. Notably, replacing catalyst C5 with C5-Me, poor results were obtained from the reaction of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylate 1a with methyl 2-[phenyl(tosylimino)methyl]acrylate 2a [Scheme 5C]. These results indicated that the free hydroxy group of catalyst C5 is essential to achieve high efficiency and asymmetric induction.
As shown in Scheme 6, the absolute configuration of the enantioenriched 1,2,3,4-tetrahydroquinoline-3-carboxylate 3aa was determined by ECD (see Supplementary Materials for details). Accordingly, a possible reaction mechanism was proposed in Scheme 7. The initial nucleophilic addition of C5 to 2-(2-phenyl-4H-benzo[d][1,3]oxazin-4-yl)acrylate 1a to form the key chiral amine-dipole intermediate I. Then, methyl 2-[phenyl(tosylimino)methyl]acrylate 2a was activated and arranged spatially by hydrogen-bond interaction to react with the chiral amine-dipole to generate 1,4-adduct intermediate II. The subsequent asymmetric intramolecular conjugate addition afforded cycloaddition product intermediate III, followed by the removal of organocatalyst C5 to re-generate the catalyst and afford the desired product 3aa.
Scheme 6. Comparison of the calculated ECD of compound (3S,4R)-3aa with the experimental one of compound 3aa.
CONCLUSIONS
In conclusion, this work demonstrated the in situ formation of chiral amine-dipoles from
DECLARATIONS
AcknowledgmentsWe gratefully thank the assistance of SUSTech Core Research Facilities, Yang Yu (HRMS, SUSTech). Computational work was supported by the Center for Computational Science and Engineering at SUSTech, and the CHEM high-performance supercomputer cluster (CHEM-HPC) located at the Department of Chemistry, SUSTech.
Authors’ contributionsDesigning the experiments, writing the manuscript, and being responsible for the whole work: Li P
Performing the experiments: Wang T
Synthesizing the substrates and data review: Wang T, Chen X, Wan Q
Determining the absolute configuration of product 3aa: Shen B, Yu P
Availability of data and materialsDetailed experimental procedures and spectroscopic data were published as Supplementary Materials in the journal.
Financial support and sponsorshipThe authors acknowledge the financial support from National Natural Science Foundation of China (21871128), Guangdong Innovative Program (2019BT02Y335), and the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Nammalwar B, Bunce RA. Recent syntheses of 1,2,3,4-tetrahydroquinolines, 2,3-dihydro-4(1
2. Zhang X, You SL. THQphos in Ir-catalyzed asymmetric allylic substitution reactions. Chimia 2018;72:589-94.
3. Sridharan V, Suryavanshi PA, Menéndez JC. Advances in the chemistry of tetrahydroquinolines. Chem Rev 2011;111:7157-259.
4. Fochi M, Caruana L, Bernardi L. Catalytic asymmetric aza-Diels-Alder reactions:the Povarov cycloaddition reaction. Synthesis 2014;46:135-57.
5. Muthukrishnan I, Sridharan V, Menéndez JC. Progress in the chemistry of tetrahydroquinolines. Chem Rev 2019;119:5057-191.
6. El-Shahat M. Advances in the reduction of quinolines to 1,2,3,4-tetrahydroquinolines. J Heterocyclic Chem 2022;59:399-421.
7. Lemos BC, Filho EV, Fiorot RG, Medici F, Greco SJ, Benaglia M. Enantioselective Povarov reactions:an update of a powerful catalytic synthetic methodology. Eur J Org Chem 2022:e202101171.
8. Tian Y, Duan M, Liu J, Fu S, Dong K, Yue H, Hou Y, Zhao Y. Recent advances in metal-catalyzed decarboxylative reactions of vinyl benzoxazinanones. Adv Synth Catal 2021;363:4461-74.
9. Chen ZC, Chen Z, Du W, Chen YC. Transformations of modified Morita-Baylis-Hillman adducts from isatins catalyzed by Lewis bases. Chem Rec 2020;20:541-55.
10. Moyano A, Rios R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem Rev 2011;111:4703-832.
11. Xie P, Huang Y. Morita-Baylis-Hillman adduct derivatives (MBHADs):versatile reactivity in Lewis base-promoted annulation. Org Biomol Chem 2015;13:8578-95.
12. Zhong N-J, Wang Y-Z, Cheng L, Wang D, Liu L. Recent advances in the annulation of Morita-Baylis-Hillman adducts. Org Biomol Chem 2018;16:5214-27.
13. Lu S, Ong JY, Poh SB, Tsang T, Zhao Y. Transition-metal-free decarboxylative propargylic substitution/cyclization with either azolium enolates or acyl anions. Angew Chem Int Ed 2018;57:5714-9.
14. Cheng Y, Han Y, Li P. Organocatalytic enantioselective [1 + 4] annulation of Morita-Baylis-Hillman carbonates with electron-deficient olefins:access to chiral 2,3-dihydrofuran derivatives. Org Lett 2017;19:4774-77.
15. Chen X, Wang T, Lu Z, Li P. Organocatalytic enantioselective formal (4 + 2)-cycloadditions of phosphine-containing dipoles with isocyanates. Org Lett 2022;24:3102-06.
16. Liu H, Zhang Q, Wang L, Tong X. PPh3-catalyzed [2 + 2 + 2] and [4 + 2] annulations:synthesis of highly substituted 1,2-dihydropyridines (DHPs). Chem Commun 2010;46:312-4.
17. Zhou B, Yuan Z, Yu J, Luan X. Dearomatization/Deiodination of
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wang T, Shen B, Chen X, Wan Q, Yu P, Li P. Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles. Chem Synth 2023;3:9. http://dx.doi.org/10.20517/cs.2022.44
AMA Style
Wang T, Shen B, Chen X, Wan Q, Yu P, Li P. Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles. Chemical Synthesis. 2023; 3(2): 9. http://dx.doi.org/10.20517/cs.2022.44
Chicago/Turabian Style
Wang, Tao, Boming Shen, Xuling Chen, Qianran Wan, Peiyuan Yu, Pengfei Li. 2023. "Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles" Chemical Synthesis. 3, no.2: 9. http://dx.doi.org/10.20517/cs.2022.44
ACS Style
Wang, T.; Shen B.; Chen X.; Wan Q.; Yu P.; Li P. Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles. Chem. Synth. 2023, 3, 9. http://dx.doi.org/10.20517/cs.2022.44
About This Article
Special Issue
Copyright
Author Biographies






Data & Comments
Data
 Cite This Article 32 clicks
Cite This Article 32 clicks



![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.1.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.2.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.3.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.4.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.5.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.6.jpg?image_process=resize,s_450)
![Organocatalytic regio- and enantioselective formal [4 + 2]-annulation of chiral nitrogen-containing dipoles](https://image.oaes.cc/b9b30f0a-ea14-42f0-9762-04cc46829046/5421.scheme.7.jpg?image_process=resize,s_450)











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.