Boosting lithium-selenium batteries
Lithium-selenium (Li-Se) batteries have been recognized as one of the most promising candidates for next-generation energy storage devices due to their high energy density and high conductivity of Se[1,2]. However, the shuttle effect has become a major shackle restricting their commercial development[3,4]. Confining active material selenium inside the cathode is an effective method to suppress this phenomenon[5]. Moreover, rapid transportation of electrons and ions is indispensable for achieving high electrochemical performance Li-Se batteries[6].
Combining the microporous carbon particles that provide Se loading space and adsorption to polyselenides with a highly conductive meso/macropores network is a design direction to suppress the shuttle effect and achieve high reaction kinetics[7]. Microporous-rich metal-organic framework (MOF) and high conductivity CNTs become the ideal compositions. The current work presents tunable size zeolitic imidazolate framework-8 (ZIF-8) derived microporous carbon particles strung by MWCNTs (ZIF-8-C@MWCNTs-X,
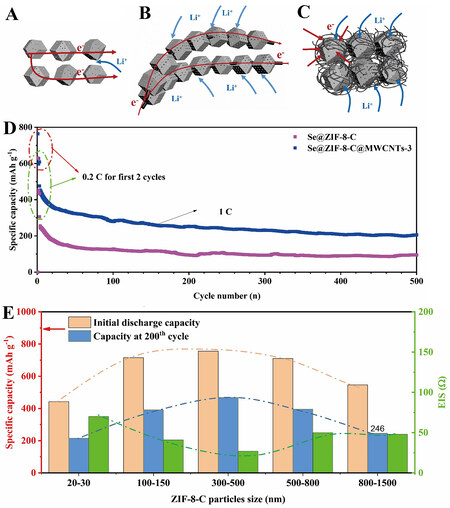
Figure 1. Schematic mechanism of the (A) Se@ZIF-8-C, (B) ZIF-8-C@MWCNTs-1 and (C) Se@ZIF-8-C@MWCNTs-5 electrodes for lithium ion and electron movement. (D) Cycling performance of Se@ZIF-8-C and Se@ZIF-8-C@MWCNTs-3 at high current density of
Compared with Se@ZIF-8-C cathode battery, the electrochemical performance of Se@ZIF-8-C@MWCNTs achieves high improvement due to the accelerated electron/ions transfer brought by the interconnected MWCNTs. Moreover, the size of ZIF-8-C particles is a critical factor for electrochemical kinetics in the charge/discharge process. For too small particles of ZIF-8-C, the problem of shuttle effect is serious due to the easily diffused out polyselenide. Otherwise, too large particle size leaves the ions transfer difficult. At an optimized particle size of 300-500 nm, both the suppressed shuttle effect and the rapid ions transfer are achieved. Se@ZIF-8-C@MWCNTs-3 with the optimized ZIF-8-C size exhibited the best cycle stability with a high capacity of 468 mAh g-1 at 0.2 C after 200 cycles. Even at the high rate of 1 C, the capacity of Se@ZIF-8-C@MWCNT-3 can remain at 206 mAh g-1 after 500 cycles.
In the designed Se@ZIF-8-C@MWCNTs system, ZIF-8-C particle functions as a reaction block, where the Se confined inside of the micropores undergoes a rapid electrochemical reaction. While the interconnected MWCNTs works as transfer block to ensure the supply of required electrons and ions. This kind of block mode design realizes both the confinement of the reaction and the rapid mass transportation. Compared with the conflated design system, the modular system enables the electrochemical reaction inside the battery to proceed in an orderly manner as living organisms. The efficient synergy of different modules is the premise for the system to maximize its value, which is reflected in the particle size of ZIF-8-C nanocarbons in the Se@ZIF-8-C@MWCNTs system. The Se@ZIF-8-MWCNTs system obtains the best match at the size of 300-500 nm.
The design concept of coordinated development of work blocks has a significant effect on the working mechanism of the system. It could give a guiding for other reaction environments. The strategy in this work realized the in-situ interconnection of the ZIF-8 derived reactor and MWCNTs highways. The effect of particle size on the reaction kinetics provides suggestions for further structural design and high performance Li-Se batteries.
DECLARATIONS
Author’s contributionThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Jin Y, Liu K, Lang J, et al. High-energy-density solid-electrolyte-based liquid Li-S and Li-Se batteries. Joule 2020;4:262-74.
2. Sun J, Du Z, Liu Y, et al. State-of-the-art and future challenges in high energy lithium-selenium batteries. Adv Mater 2021;33:e2003845.
4. Song JP, Wu L, Dong WD, et al. MOF-derived nitrogen-doped core-shell hierarchical porous carbon confining selenium for advanced lithium-selenium batteries. Nanoscale 2019;11:6970-81.
5. Lei Y, Liang X, Yang L, et al. Li-Se batteries: insights to the confined structure of selenium in hierarchical porous carbon and discharge mechanism in the carbonate electrolyte. Carbon 2022;191:122-31.
6. Li C, Wang Y, Li H, et al. Weaving 3D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li-Se battery. J Energy Chem 2021;59:396-404.
7. Wang X, Tan Y, Liu Z, et al. New insight into the confinement effect of microporous carbon in Li/Se battery chemistry: a cathode with enhanced conductivity. Small 2020;16:e2000266.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yao X. Boosting lithium-selenium batteries. Chem Synth 2022;2:10. http://dx.doi.org/10.20517/cs.2022.13
AMA Style
Yao X. Boosting lithium-selenium batteries. Chemical Synthesis. 2022; 2(2): 10. http://dx.doi.org/10.20517/cs.2022.13
Chicago/Turabian Style
Yao, Xiangdong. 2022. "Boosting lithium-selenium batteries" Chemical Synthesis. 2, no.2: 10. http://dx.doi.org/10.20517/cs.2022.13
ACS Style
Yao, X. Boosting lithium-selenium batteries. Chem. Synth. 2022, 2, 10. http://dx.doi.org/10.20517/cs.2022.13
About This Article
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.