Mn-Ce catalysts for highly efficient C-H activation
Keywords
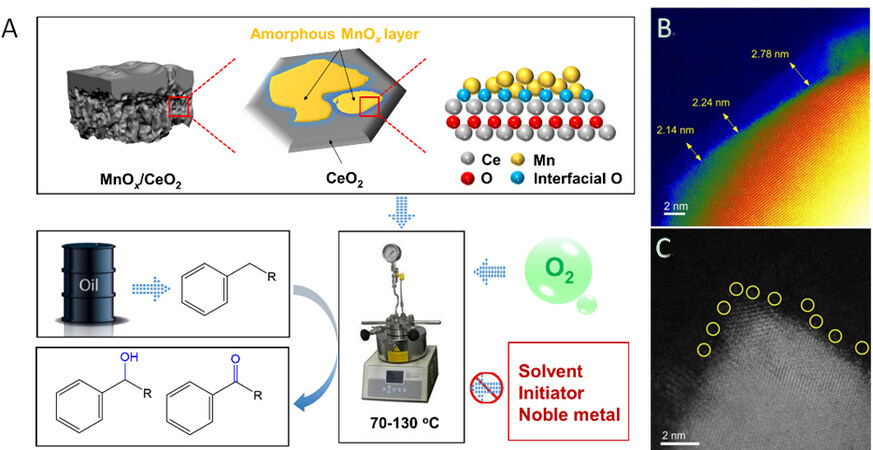
The environmentally friendly and effective oxidation of sp3-hybridized C-H bonds has attracted much attention for the sustainable production of alcohols/ketones/epoxides from petroleum hydrocarbons[1,2]. However, the catalysis with oxygen as the oxidant is obstacled by the low efficacy of the catalysts. Mn-Ce solid-solution is reported as a promising catalyst for the aerobic oxidation reactions because of its maximized Mn-O-Ce interfaces, but expensive surfactants and ionic liquids and high Mn content are usually required for maximization of solid-solution phase, which is really costly for the catalyst preparation[3]. However, for oxidation reactions, the solid solution phase is not always the most efficient catalyst[4]. The efficient catalytic oxidation of C-H bonds with molecular oxygen over non-noble metal catalysts, as well as avoiding the solvents and initiator additives, is highly desired yet challenging. Titania (TiO2) is one of the preferred strong oxide-support interactions (SOSIs) supports[5], but Wang et al.[6] reported a highly active Mn-Ce oxide catalyst with SOSIs for the catalytic partial oxidation of hydrocarbons without solvents and initiator additives [Figure 1A]. The TEM analysis showed the existence of manganese oxide layers with two-dimensional and amorphous status on the cerium matrix [MnOx/CeO2, Figure 1B and C], and a high capacity of active oxygen species at 46.1% is obtained. The MnOx/CeO2 catalyst with SOSIs shows high activities and selectivities in the oxidation of hydrocarbons to alcohols and ketones with molecular oxygen under solvent- and initiator-free conditions at mild temperature [Figure 1A], which outperform the noble metal catalysts and the highly efficient solid-solution Mn-Ce catalysts[3,7]. Importantly, the MnOx/CeO2 is stable and exhibits constant performances in the continuous recycling tests.
Figure 1. (A) Synthetic and catalytic strategies of the ceria-supported two-dimensional manganese oxide catalyst with SOSIs. (B,C) High-resolution STEM images of MnOx/CeO2 catalyst with SOSIs. The isolated Ce sites in the amorphous region were highlighted by yellow circles in (C)[6].
Multiple characterizations (EPR, in situ Raman, in situ FTIR, in situ XPS…) are used to study the redox properties of the materials. The EPR, Raman, and XPS results demonstrated the existence of abundant oxygen vacancies/defective sites in the MnOx/CeO2 catalyst, which are beneficial for oxygen activation. More importantly, the in situ Raman and XPS studies indicated the availability of the active oxygen species at low temperature (< 80 °C) and can be regenerated during the continuous oxidation process, which makes it extremely active for the aerobic oxidation reactions. The in situ FTIR experiments confirmed the pivotal role of surface active oxygen species for the adsorption and activation of the C-H bond, which accelerated the conversion of hydrocarbons to corresponding alcohols and/or ketones.
This work teaches us how to use the SOSIs to construct abundant active interfacial sites for the activation of C-H bonds. The interfacial sites can not only regulate the catalytic activities but also foster further studies on the structure-performance relationship of various supported metal oxide catalysts. It is anticipated that this method will be further applied for designing efficient metal oxide catalysts for the sustainable production of valuable chemicals.
DECLARATIONS
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there is no conflict of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. Marimuthu A, Zhang J, Linic S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 2013;339:1590-3.
3. Zhang P, Lu H, Zhou Y, et al. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons. Nat Commun 2015;6:8446.
4. Jonas F, Lebeau B, Siffert S, et al. Nanoporous CeO2-ZrO2 Oxides for Oxidation of Volatile Organic Compounds. ACS Appl Nano Mater 2021;4:1786-97.
5. Barakat T, Rooke JC, Cousin R, et al. Investigation of the elimination of VOC mixtures over a Pd-loaded V-doped TiO2 support. New J Chem 2014;38:2066-74.
6. Wang H, Wang L, Luo Q, et al. Two-dimentional manganese oxide on ceria for the catalytic partial oxidation of hydrocarbons. CS 2022; doi: 10.20517/cs.2022.02.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Siffert S. Mn-Ce catalysts for highly efficient C-H activation. Chem Synth 2022;2:6. http://dx.doi.org/10.20517/cs.2022.07
AMA Style
Siffert S. Mn-Ce catalysts for highly efficient C-H activation. Chemical Synthesis. 2022; 2(1): 6. http://dx.doi.org/10.20517/cs.2022.07
Chicago/Turabian Style
Siffert, Stephane. 2022. "Mn-Ce catalysts for highly efficient C-H activation" Chemical Synthesis. 2, no.1: 6. http://dx.doi.org/10.20517/cs.2022.07
ACS Style
Siffert, S. Mn-Ce catalysts for highly efficient C-H activation. Chem. Synth. 2022, 2, 6. http://dx.doi.org/10.20517/cs.2022.07
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.