Recent advances in radical phosphorylation
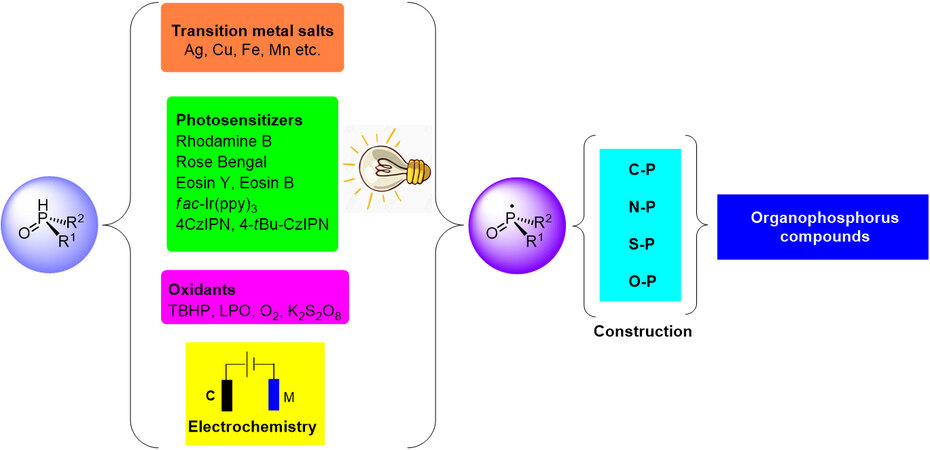
As an important class of chemical molecules, organophosphorus compounds play an irreplaceable role in human industry and life[1-3]. Therefore, the construction of phosphorus-containing compounds has attracted significant attention from organic chemists. In recent years, there have been advances made beyond the traditional C-P bond formation strategy such as the Arbuzov reaction, Hirao reaction, Friedel-Crafts-type reaction of PCl3 with aromatic compounds, and the reactions of organometallic reagents with electrophilic phosphorus species[4,5]; the construction of X-P (X=C, N, O, or S) bonds with P-centered radicals has created a new avenue for the construction of organophosphorus compounds [Figure 1][6-11]. In the latest issue of Chemical Synthesis, Liu et al.[12] reviewed the recent advances in transition metal-catalyzed, photocatalyzed, and electrocatalyzed C-P, N-P, S-P, and O-P bond formation based on P-centered radicals in the past five years, and some earlier pioneering works were also discussed.
In the review, Liu et al.[12] first briefly introduced the geometries of P-centered radicals and general methods for generating P-centered radicals and also exhibited some common structures of photocatalysts used in phosphorylation reactions. Subsequently, the authors described in detail the radical functionalization of alkenes based on P-centered radicals with different strategies including visible light photoredox-catalyzed reactions and transition metal-catalyzed reactions. In the visible light photoredox-catalyzed reactions, in addition to traditional metal photosensitizers, some organic dyes like Eosin Y can also promote this kind of reaction as well. This summary could play a certain guiding role in the subsequent reaction design. Additionally, a series of P-centered radicals-based difunctionalization of alkenes were also discussed in the section.
Liu et al.[12] then summarized the radical phosphorylation of alkynes. In addition, the authors also focused on the discussion of difunctionalized phosphorylation of alkynes. Furthermore, π-conjugated phosphine molecules have become vitally important in the blooming area of organic light-emitting diodes and photovoltaic cells. The radical cascade cyclization phosphorylation of alkynes has also been well developed and was included in this review. Moreover, arylphenylisonitriles, which contain a C≡N π-bond and have good radical acceptor properties, were also compatible with radical phosphorylation though visible light-induced cascade reaction.
Liu et al.[12] continued to introduce the cross-coupling phosphorylation reactions of (pseudo) halides via transition-metal and photoredox dual catalysis. Moreover, decarboxylative or denitro phosphorylation and dehydrogenative C-H phosphorylations were also discussed in the review.
In the final section of their review, Liu et al.[12] summarized several other types of radical phosphorylation such as C(sp3)-P bond formation via C-C and P-H bond cleavages and Atherton-Todd-type reaction. Notably, electrochemical oxidative phosphorylation with hydrogen evolution was also discussed.
In summary, Liu et al.[12] provided readers with an overview of transition metal-mediated and photoredox-catalyzed radical phosphorylation reactions. At the same time, the review also introduced the method of electrochemical phosphorylation to synthesize organophosphorus compounds. The authors summarized the latest advances on phosphorylation reaction over the past five years. Furthermore, on the basis of this review, the authors put forward their own views on phosphorylation and prospects for the future. This information provided valuable insights and guidelines for the design of such reactions.
DECLARATIONS
Authors’ contributionsPrepared the manuscript: Niu Y, Yang SD
Availability of data and materialsNot applicable.
Financial support and sponsorshipWe are grateful to the NSFC (No. 21532001) for financial support.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem Rev 2003;103:3029-70.
2. Demmer CS, Krogsgaard-Larsen N, Bunch L. Review on modern advances of chemical methods for the introduction of a phosphonic acid group. Chem Rev 2011;111:7981-8006.
3. Duffy MP, Delaunay W, Bouit PA, Hissler M. π-conjugated phospholes and their incorporation into devices: components with a great deal of potential. Chem Soc Rev 2016;45:5296-310.
4. der Jeught S, Stevens CV. Direct phosphonylation of aromatic azaheterocycles. Chem Rev 2009;109:2672-702.
5. Shao C, Xu W, Li L, Zhang X. Recent Advances of transition metal-catalyzed P-C coupling reactions. Chin J Org Chem 2017;37:335.
6. Gao Y, Tang G, Zhao Y. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur Silicon Relat Elem 2017;192:589-96.
7. Lan X, Wang N, Xing Y. Recent advances in radical difunctionalization of simple alkenes: recent advances in radical difunctionalization of simple alkenes. Eur J Org Chem 2017;2017:5821-51.
8. Gao Y, Tang G, Zhao Y. Recent advances of phosphorus-centered radical promoted difunctionalization of unsaturated carbon-carbon bonds. Chin J Org Chem 2018;38:62.
9. Cai B, Xuan J, Xiao W. Visible light-mediated C P bond formation reactions. Science Bulletin 2019;64:337-50.
10. Chen L, Liu X, Zou Y. Recent advances in the construction of phosphorus-substituted heterocycles, 2009-2019. Adv Synth Catal 2020;362:1724-818.
11. Ung SP, Mechrouk VA, Li C. Shining light on the light-bearing element: a brief review of photomediated C-H phosphorylation reactions. Synthesis 2021;53:1003-22.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Niu Y, Yang SD. Recent advances in radical phosphorylation. Chem Synth 2021;1:12. http://dx.doi.org/10.20517/cs.2021.14
AMA Style
Niu Y, Yang SD. Recent advances in radical phosphorylation. Chemical Synthesis. 2021; 1(1): 12. http://dx.doi.org/10.20517/cs.2021.14
Chicago/Turabian Style
Niu, Yuan, Shang-Dong Yang. 2021. "Recent advances in radical phosphorylation" Chemical Synthesis. 1, no.1: 12. http://dx.doi.org/10.20517/cs.2021.14
ACS Style
Niu, Y.; Yang S.D. Recent advances in radical phosphorylation. Chem. Synth. 2021, 1, 12. http://dx.doi.org/10.20517/cs.2021.14
About This Article
Copyright
Data & Comments
Data
 Cite This Article 20 clicks
Cite This Article 20 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.